Stool Examination:- Part 2 – Stool Concentration Methods, Stains, Handling, and Preservatives
Stool Examination
Sample for Stool Examination
- Can take a random stool sample.
- More than 2 grams of the stool is needed, ideally 2 to 5 grams, sometimes called a pigeon’s egg.
- To rule out worm infestation, three consecutive stools are tested.
- Collect three stools in the span of 10 days.
- Two samples on alternate days.
- The hospitalized patient can take a stool sample every day.
- Multiple samples are needed to rule out the parasitic infestation.
- One sample after purgation.
- Collect the sample in a clean, water-tight, dry, urine-free container with a tight lid.
- In the case of Infants, collect from the diaper.
Concentration methods:
- It is used for:
- Protozoan cysts and helminth eggs.
- It consists of the following:
- Sedimentation method.
- Floatation technique.
Qualitative method for fats:
- Fat in the feces is stained with Sudan III.
- Neutral fat is seen as bright orange droplets.
- Fatty acids usually do not stain.
A quantitative method for fats:
- It will quantitate fat contents in the stool in patients with steatorrhea.
- Method:
- Collect stool for 3 consecutive days.
- The patient must have a diet containing roughly 50 to 150 grams/day of fat 2 days before stool collection.
- It is important to be sure that all stool samples are collected and that the patient is not incontinent of feces.
- Patient noncompliance with complete stool collection is mostly the common cause of false negative results.
- In constipated patients, give bedtime laxatives.
Normal fat contents:
- In a normal person = <7 grams of fats in the stool/24 hours.
- Pancreatic insufficiency = >9.5 grams/100 grams of stool. (it is also seen in bacterial overgrowth or biliary tract disease).
- Nontropiocal sprue and celiac disease (24 hours fats) = <9.5 grams/100 grams of the stool favors.
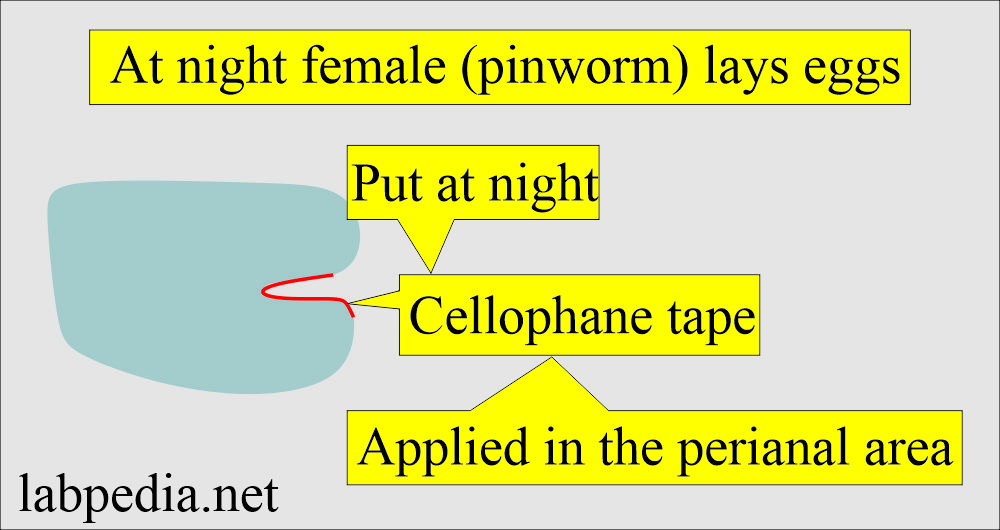
Cellophane tape method for kids for pinworms (Enterobius vermicularis):
- This is also known as Scotch tape preparation.
- This is the best method for diagnosing infant and child pinworms (Enterobius vermicularis) infestation.
- The female (pinworm) at night, when the child is resting, a female (pinworm) comes out of the rectum and lays eggs in the perianal area at night.
- Put the tape at night and collect the cellophane tape in the morning.
Procedure for pinworms:
- Take 10 cm of the transparent adhesive tape.
- Fold it on the tongue depressor with the adhesive side out.
- Now press the adhesive side of the tape over the perianal area and cover the maximum area. Should apply this tape at night.
- In the morning, remove the tape and put the adhesive side on the slide.
- See under the microscope.
Methods to prepare the stool smears:
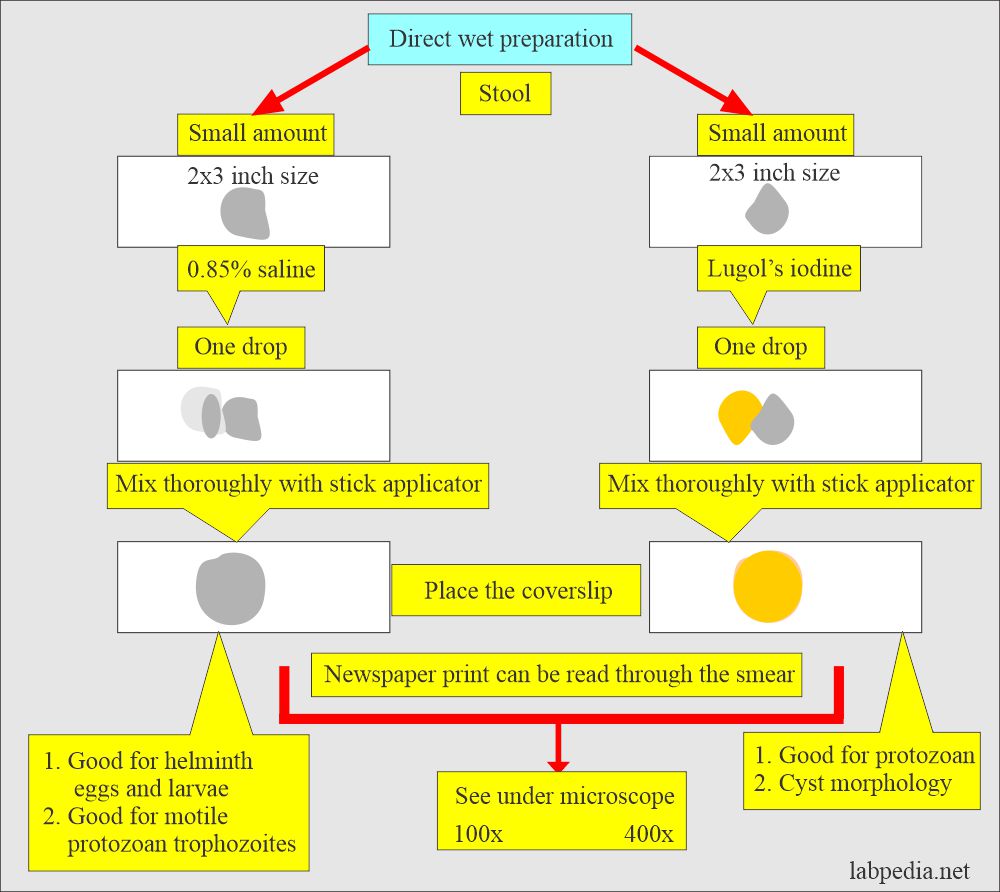
Saline wet preparation:
- Take one drop of 0.85% saline.
- Take a small amount of stool and mix well.
- The smear should be thin to see the newsprint under the slide.
- Put cover glass and see under the microscope 100x and 400x objective.
- This is best to see helminth eggs, larvae, and trophozoites.
Iodine wet preparation:
- This is also called wet preparation.
Lugol’s iodine solution preparation:
Iodine solution consists of:
- Potassium iodide (KI) = 10 grams
- Iodine powder crystals = 5 grams
- Distilled water = 100 ml
Procedure to make Lugol’s iodine solution:
- Dissolve KI in D.water.
- Slowly add iodine crystals.
- Shake the solution gently until they dissolve.
- Filter the solution before use, and this is the stock solution.
- Dilute the stock solution 1:5 with D. water. Make this working solution before use.
How to make stool smear with Lugol’s Iodine:
- Take a drop of Lugol’s iodine solution.
- Take a small amount of stool and mix it well.
- Make a thin smear.
- Put the cover glass on it and gently press it to get an evenly thin smear.
- See under 100 x and 400 x objective lenses.
- Too weak iodine solution; in that case, organisms will not stain properly.
- Too strong an iodine solution will clump the stool.
Concentration methods for stool:
- Purpose of the concentration method:
- The main aim of the concentration method is to remove debris.
- Also, when the parasite is low in number.
There are three methods used for the concentration of stool:
- Formalin-ethyl-acetate concentration method.
- Zinc-floatation method.
- Sheather sugar floatation method.
1. The formalin-ethyl-acetate concentration method for stool:
- The formalin-ethyl-acetate concentration method is most commonly used.
- This method recovers the helminth eggs and larvae, to a lesser extent, trophozoites.
Principle: This is based on specific gravity. After centrifugation, the stool’s parasites are heavier and settle at the bottom as sediments.
Debris is lighter and rises to the upper layers.
Advantages are:
- It is easy to prepare the solution.
- This is inexpensive.
- The procedure is easy to perform.
- There is a rare distortion of parasite forms (eggs).
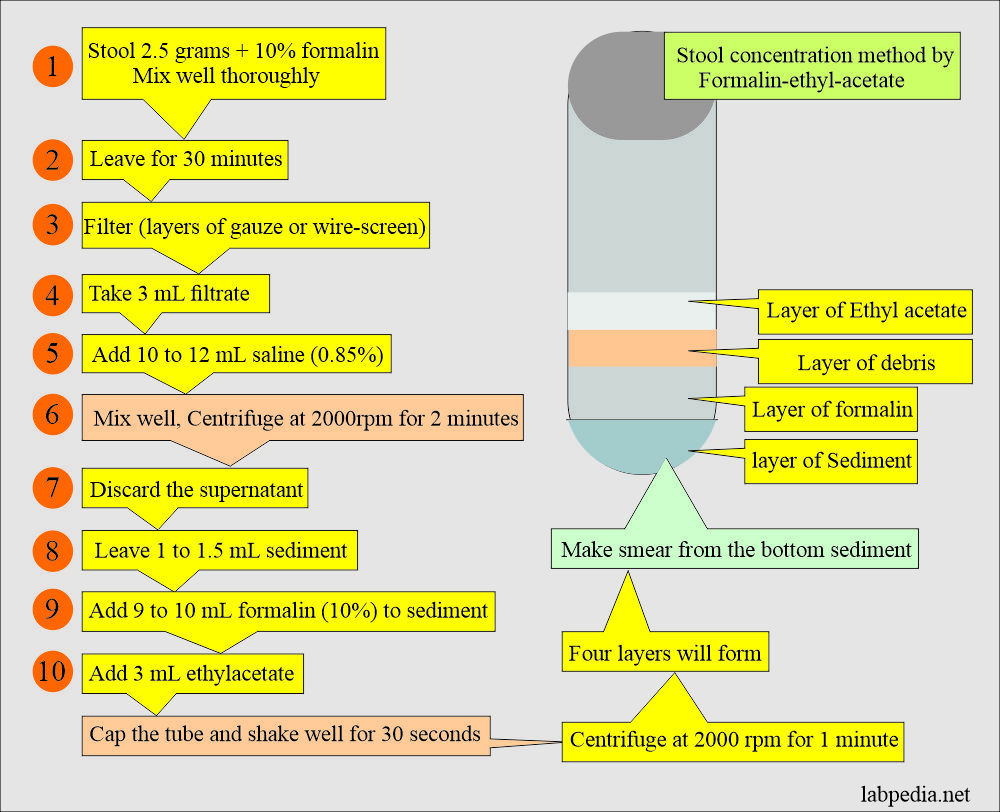
2. The procedure of formalin-ethyl-acetate concentration:
- The stool should be fixed in formalin for at least 30 minutes.
- Mix 2 to 5 grams of the stool thoroughly in the 10% formalin.
- Filter the above stool in the formalin.
- This can be done by two layers of gauze or a wire screen and collecting around 3 mL.
- Add 10 to 12 mL of 0.85% saline and mix it well.
- Centrifuge for 2 minutes at 2000 RPM (or 2500 RPM).
- Discard the supernatant and leave 1 to 1.5 mL of the sediment.
- If the supernatant is cloudy, then repeat the above steps of saline.
- Add 9 mL of 10% formalin to the sediment.
- Now add 3 mL of ethyl acetate.
- Cap the test tube and shake well for 30 seconds.
- Centrifuge the tubes for 1 minute at 2000 RPM.
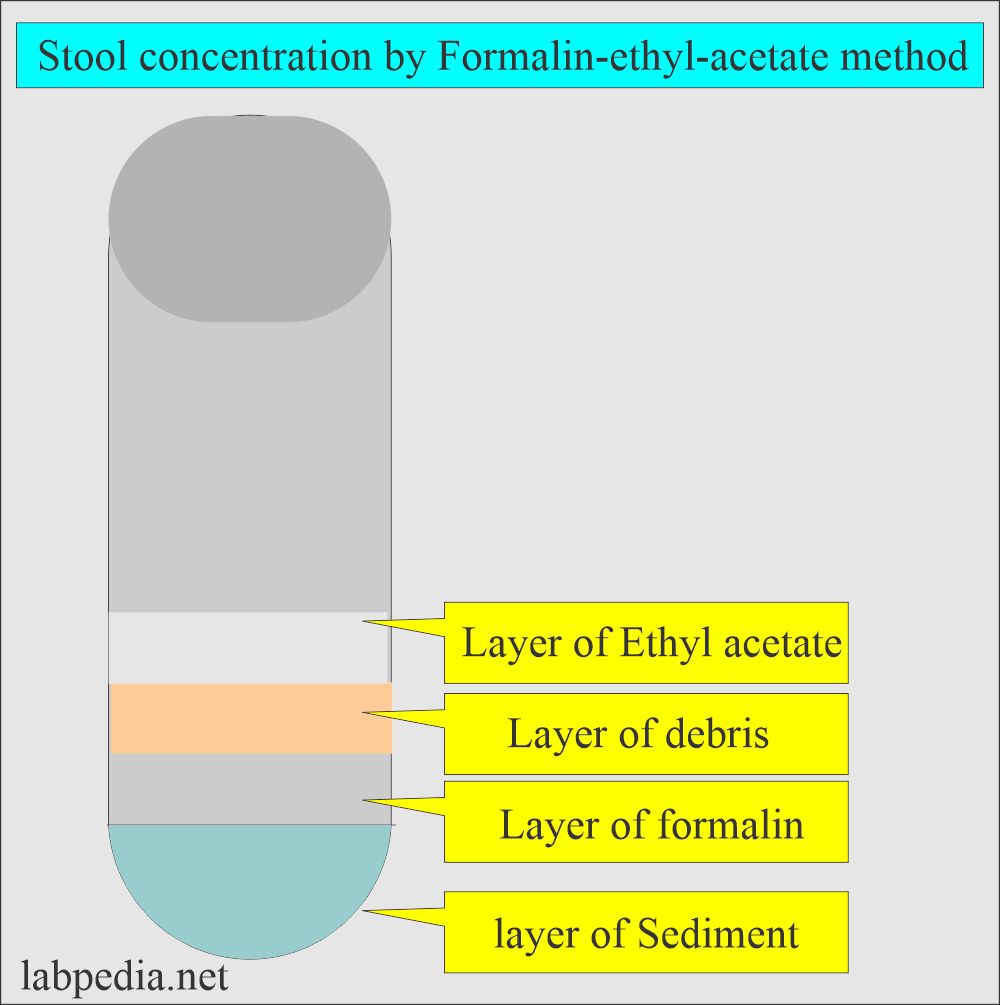
- Four layers will form. The bottom is the sediment that is needed to prepare the smear.
- Remove the debris with a wooden applicator stick. Decant the upper three layers carefully and leave the sediments in the test tube.
- Clean the sides of the test tube with a swab.
- Giardia cyst may stick to the side of the test tube.
- Add a few drops of the formalin and mix the sediment thoroughly. This will preserve the sediment.
- Now, we can make the smears in saline and iodine wet preparation.
- Examine under the microscope.
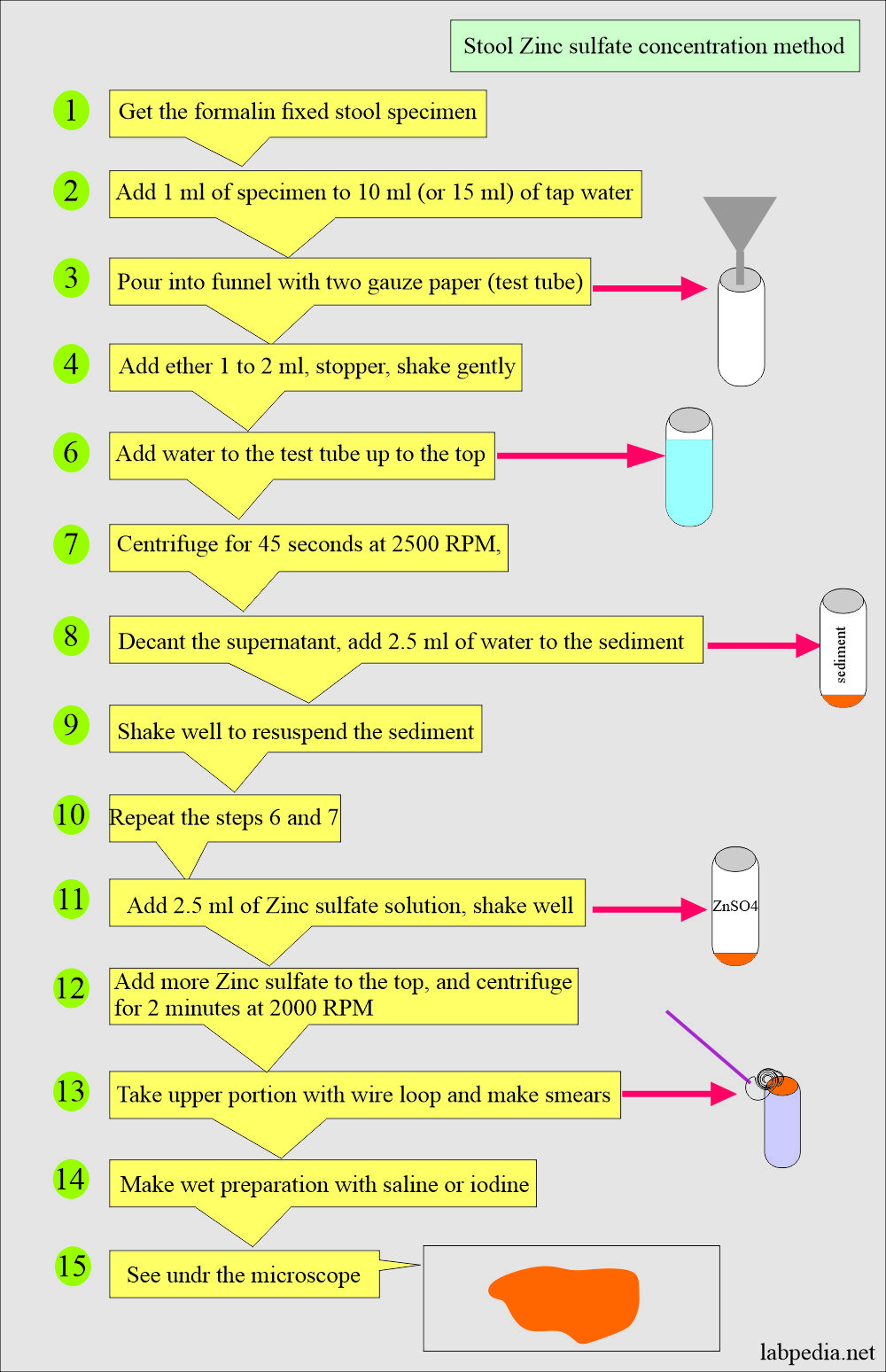
3. Zinc sulfate floatation method:
- Some authors believe it is superior for concentrating and identifying eggs and protozoan cysts.
- The parasites are lighter and float on the surface, while the debris settles at the bottom.
The procedure of Zinc sulfate floatation:
- Fix the stool in the formalin.
- Make a dilution of the above specimen (1 mL) with tap water from 1:10 to 15.
- Pour the above suspension through a funnel with two layers of gauze in a small test tube.
- Add 2 mL of ether to the test tube with a stopper and gently shake.
- Now add water to the above test tube to the top just 1 cm from above.
- Centrifuge at 2500 rpm for 45 seconds.
- Decant the supernatant.
- Add 2.5 ml of the water to the sediment, shake well to resuspend the sediment—repeat steps 5 and 6.
- Add 2.5 ml of zinc sulfate (ZnSO4) to the sediment to resuspend it.
- Add zinc sulfate solution to the top of the test tube, leaving only 1 to 0.5 cm open on top.
- Centrifuge the test tube for 2 minutes at 2000 RPM.
- Take the surface material with a wire loop; this is the material where you can see the parasites.
- Make wet preparation with saline and iodine.
- Examine under the microscope.
Collection, Precautions, and Handling of the Stool samples
Precautions before the collection:
- Advise patients about the following things for at least 48 hours before the collection of the stool:
- Avoid mineral oils.
- Do not take bismuth.
- Don’t take antibiotics like tetracyclines.
- Don’t take anti-diarrheal drugs that are non-absorbent.
- Avoid anti-malarial drugs.
- The patient should not have a barium swallow examination before the stool examination.
- Stop iron-containing drugs, meat, and fish 48 hours before the collection of occult blood.
- For antibiotics or contrast media, either take a sample before or after one week.
- These substances produce unknown objects or mask the parasites.
- Warm stools are better for the ova and parasites.
- Don’t refrigerate the stool for ova and parasites.
- Stools for ova and parasites can be collected in formalin and polyvinyl alcohol. These are used as a fixative.
- If there is blood or mucus, that should be included in the stool because most of the pathogens are found in this substance.
- Examine the stool before giving antibiotics or other drugs.
- The semi-formed stool should be examined within 60 minutes of collection.
- The liquid stool should be examined within the first 30 minutes.
- The solid stool should be examined within the first hour of collection.
- Trophozoites degenerate in liquid stool rapidly, so examine the stool within 30 minutes.
- In the case of constipated cases, use non-residual purgative on the night before collecting the stool.
- Avoid urine contamination because some of the parasites are destroyed by the urine.
- Water destroys some of the parasites like Schistosome eggs and amoebic trophozoites.
- Toilet paper in the stool makes it difficult to examine the stool and may mask the parasites.
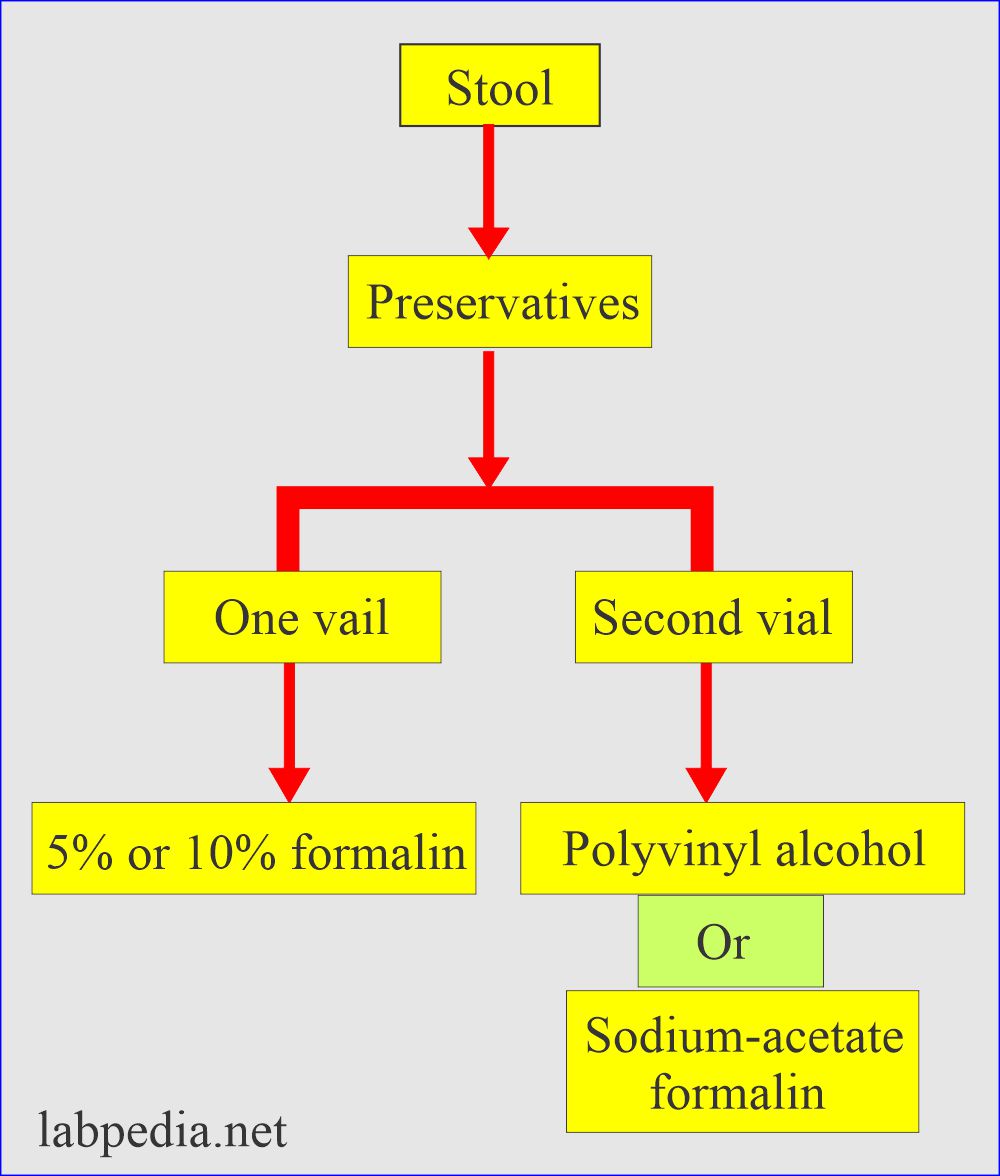
Stool preservatives are:
- In routine, stool preservatives used are:
1. Formalin for stool:
- 5% is ideal for protozoan cysts.
- 10% preserves eggs and cysts.
- Advantages are:
- It is easy to prepare.
- It can preserve the stool for several years.
- It has a long shelf life.
- Disadvantages are:
- It is not good for a permanent smear.
- Details of eggs and cysts fade away.
- Trophozoites can not be recovered.
2. Polyvinyl alcohol for stool:
- This is an effective parasitic fixative.
- This can be used along with Schaudinn’s solution.
- Advantages are:
- This is easy to do with stool.
- This helps in gluing the sample on the slide.
- It has a long shelf life when stored at room temperature.
- Smears can stain with trichrome and, iron, hemotoxylin.
- Disadvantages are:
- A large amount of mercury is present in the solution, which is hazardous to health.
- It is not easy to prepare in the lab.
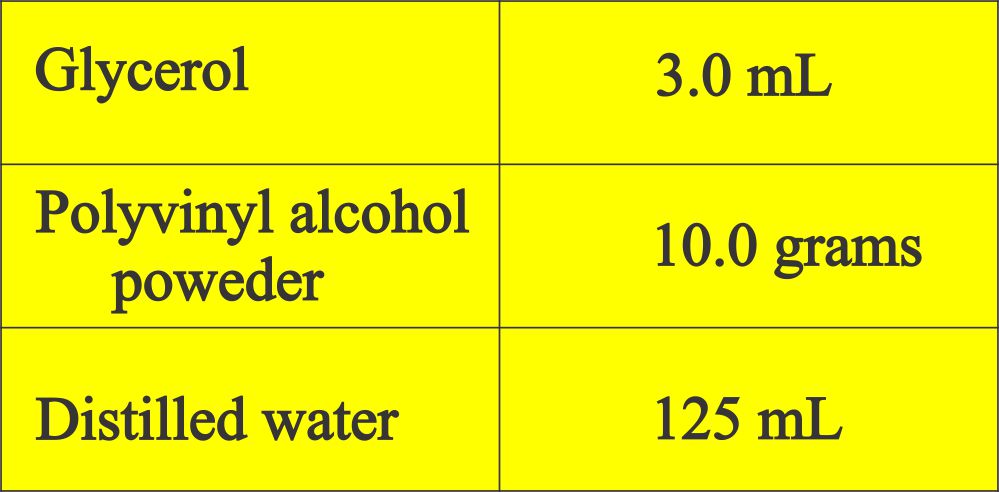
The formula of Polyvinyl alcohol:
- Procedure: Add glycerol to PVA in a large beaker.
-
- Blend it with a glass rod by stirring.
- Gradually add water and keep on mixing.
- Leave it overnight before use.
-
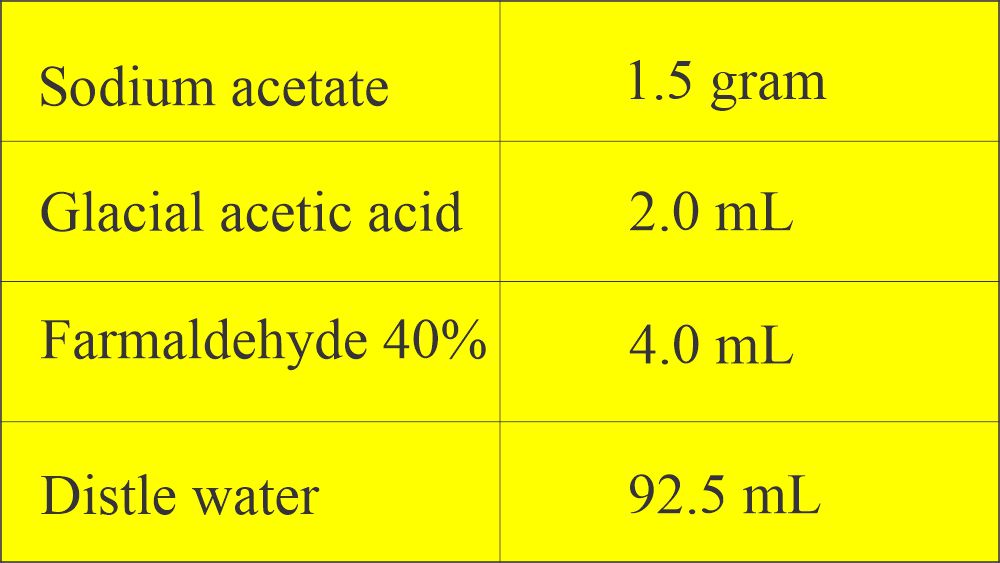
3. Sodium-acetate formalin mixture (SAF)
- There is increased interest in the use of this fixative.
- Advantages are:
- This is good for intestinal protozoa and coccidia-like bodies.
- This fixative eliminates the use of mercury compounds.
- This is an inexpensive fixative.
- This is easy to prepare in the lab.
- It has a buffering effect to decrease the distortion of the protozoa.
- This can be used in a concentration of the stool smear.
- It has good results when used with iron and hematoxylin permanent stain.
- Disadvantages are:
- This does not have adhesive properties so you may need albumin.
- It is diluted with water.
- The formula of Sodium acetate formalin:
- If the sample needs delay, use stool preservatives; otherwise, reject the sample.
- Send sample in two vials:
- One contains 5% or 10% formalin.
- The second vial contains either polyvinyl or sodium acetate formalin.
4. Preservatives for the wet preparation are:
- 10% formol-saline for the wet preparation. This is the best preservative as it kills bacteria and preserves protozoa and helminths.
- Another preservative is Sodium acetate formalin (SAF).
- Methionate iodine formalin. This is a good preservative for the field collection of the stool.
- For staining, use Polyvinyl alcohol.
- Avoid preservatives for the culture of stool.
- Usually, three parts of the preservatives and one part of the stool are used.
The permanent stain of the stool smears:
The sample of choice for stains is a thinly prepared slide from a PVA preservative (polyvinyl alcohol).
There are three methods for permanent stain:
- Wheatley trichrome.
- Iron hematoxylin.
- Modified acid-fast stain.
- The most commonly used is the Wheatley trichrome.
Wheatley trichrome procedure:
- Take the following Coplin jar and put the chemicals in those jars:
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | |
| Reagents | Iodine +70% alcohol | 70% alcohol | 70% alcohol | Trichrome stain | 90% acidified alcohol | 95% alcohol | 95% alcohol | Carboxylene | Xylene |
| Purpose of the reagents | remove the HgCl and hydration | remove iodine and hydration | Rinse, hydration | Stain | Destain | Stop staining | Dehydration | Removes debris and dehydration | Removes debris |
| Time for each jar | 10 minutes | 5 minutes | 5 minutes | 7 minutes | 5 to 10 seconds | Quick rinse | 5 minutes | 10 minutes | 10 minutes |
- Now seal the slide with the fixative and keep it for some time.
- Examine the slide under the oil immersion lens.
Questions and answers:
Question 1: What is the purpose of the concentration of the stool.
Question 2: What is the best way to get samples from the children in case of pinworm infestation.

Thank you.
Thanks for the encouraging comments.
Thanks so much
Great for the encouraging remarks
very nice presentatation.
Thanks for the encouraging remarks.
I need this stool examination book
This is a comprehensive study on the stool. Please consult this website labpedia.net
There is no such book like this on the stool. This is my own research and I have put it on website labpedia.net
It is very good note. Present in very well. I study this topic in simply and properly. It is realy usefull in all students and Others. Thank u
Thanks.
thank you for your great effort
Thanks for the encouraging remarks.
stool examination
Thanks
stool examination good
Thanks
Can i do the test with just saline and no preservatives? Also, can i use the sedimentation method(leave the tube in room temperature for an hour or 40 minutes then discard the supernatant and examine the sediment)? Thank u so much i like your article it is very helpful.
Thanks for the question. The purpose of the preservative is to preserve the ova and parasite. In saline, there may be deterioration.You can use a concentrated saline solution and then make the smear from the top layer. Moreover you can do practically experiment and see the results.
thank you dr diaz, very helpful
i mean *dr riaz
Thanks
Thanks for the encouraging remarks
I normally examine using ×10 and cover slip when using×40….otherwise nice presentation.
I think screening in x1o is the best option if you are experienced to find ova, cyst, and parasite. While x40 needed to confirm the diagnosis.
I agree with you, thanks.
Nice compilation.
THANK YOU. Sir!
Thanks for the encouraging remarks
Thanks so much
Dr. If I find Bacteria in stool smear microscopically and I don’t do Gram stain to classify the bacteria also stool culture not done,
Should I report the diagnosis as bacteria Infection and being clinical manifestation which needs Treatment?
I don’t think so because normally you can see the bacteria. You should get the history of the patient, and also you will see WBcs and maybe a few RBCs. The presence of WBCs indicates infection. Only bacteria may be ignored.
Thanks very much .
so complete so useful .
Thanks for the encouraging remarks.
Dr. Riaz, Thank you all your information. You made it easier to understand.
Thanks for the comments.
Thank you for your efforts to create this website, I have been searching for long time for such easy access to any lab topics, previously I have to go to some text books for any info about lab. This will help me in diagnosing any samples as i am a lab technician. I would like you to improve further.
Thanks for the comments. Actually we are continously improving the various topics.
Nice one kindly email me the pdf [email protected]
Thanks. Please let me know which topic pdf you want.
May God bless you for this articles,lets me be together with you for more research.
Thanks a lot. You are welcome and let me know how you can help and improve it.
Thank you so much. The article has answered all my questions.
Thanks, we are trying our best to update and add more material to each subject.
Thank you so much,i understand and learn more and more in this lesson keep it up
Thanks. We are updating most of the topics.
Thank you so much for this site. I am a pathology technician and this is quite informative and helpful in my forthcoming interview. Thanks a lot again.
Thanks. You are welcome.
I agree with you
Thanks.
I like this work well analyzied
Thanks.
Dr. Riaz, thanks for your endeavor
Myself also Reaz
Really a nice piece of work, actually what I am looking for
Thanks.
Can you please refer any ideal reference book for stool examination?
Dear, There are so many reference books. I can recommend a few of these:
Medical parasitology by Markell, John, and Krotoski (W.B. Saunders Company).
Foundation of parasitology by Gerald, Schmidt, and Roberts (Wm. C. Brown publishers).
Lynch’s Medical laboratory technology by Raphael (W.B. Saunders Company).
I hope these will help you.
The topic is highly appreciable. Immeasurable thanks for the full explanation
Thanks.
nice information. thanks a lot
Thanks.
Please what’s the normal pus cell in stool?
I don’t think there is any normal number of WBC in the stool. Their presence is abnormal.
what’s the interpretation if there is presence of e.coli and pus cells in stool of an acute gastroenteritis patient?
This depends upon the age of the patients. In children, E.coli can cause diarrhea. The pus cell presence indicates infection.
thank you doc, nice and useful info
Thanks.
Very informative Article Doctor. What is the Publication date ?
Updated on October 28, 2020. Thanks for the remarks.
Good worker done God bless you
Thanks.
thank you sir for this piece it is very helpful
Thanks.
5. While doing the microscopic examination of stool specimen, you have noticed that there are many epithelial cells and few calcium crystals found per field. What action you will do?
If there are no WBCs, then no problem. Calcium oxalate crystals may be seen after taking the calcium-rich vegetables.
A 25 year old adult patient came to the laboratory to submit urine specimen. The specimen was 1 mL only. What will you do?
While doing the microscopic examination of stool specimen, you have noticed that there are many epithelial cells and few calcium crystals found per field. What action you will do?
sir Can you name other cysts seen in stool.
I am always updating the subject, soon I will add more cysts.
Ok sir 👍
Masha Allah this website is amazing 🧿
Thanks for helping us
Thanks a lot.
Really extensive, precise, and excellent information Loved it
Thanks, Dr. Dipak
Nice write- up, please I need the parasite chart
Please let me know the details of the parasite chart which you want. I will try my best for its presentation on labpedia.net
Can i report ova of stool per HPF like this Ascariasis lumbricoide ova/ HPF
I don’t think any problem in reporting ova /HPF. Also, you can report mild, moderate, and severe infestation. You have to explain to the physician what you are trying to explain.
Thanks so much, I find your article helpful
Thanks.
That was a very helpful article. Thank you.
Thanks for the appreciation.
THANKS A LOT SIR FOR INFORMATIVE ARTICLE-JAZAKALLAHKHAIR.IF THERE ARE 2- 4 RBC,IS IT SIGNIFICANT FINDING ?OCCULT BLOOD NEGATIVE IN THE SAME SAMPLE.PT HAVING H/O GI BLEED 2 MONTHS AGO.THANKS A LOT SIR.
I think 2 to 4 RBCs need further workup. Occult blood is negative. If the RBCs are intact in shape, then these may be from the rectum like hemorrhoids.
FINE SIR.THANKS A LOT FOR YOUR VALUABLE ADVISE.
thanks a lot sir
can we report bacteria in stool microscopically, with out a bacterial culture
I don’t think there is any need to report bacteria in the stool because these are normally seen in the stool.
Please Dr Riaz I would want to know more about stool microscopy and stool mcs separately.
You are welcome.
Very useful
Thank you so much for your efforts
Thanks.
Thanks for the good information. But my question is, how can I prepare 10% formalin?
Formalin provided is taken as 100%. Now take 10 mL of formalin and add 90 mL of water. It will be 10% formalin.
pls i need stool quantitative techniques
principle
procedure
Please see the link:
https://labpedia.net/stool-examination-part-2-stool-smear-preparation-stains-handling-and-preservatives/