Laboratory Glassware, cleaning and Sterilization
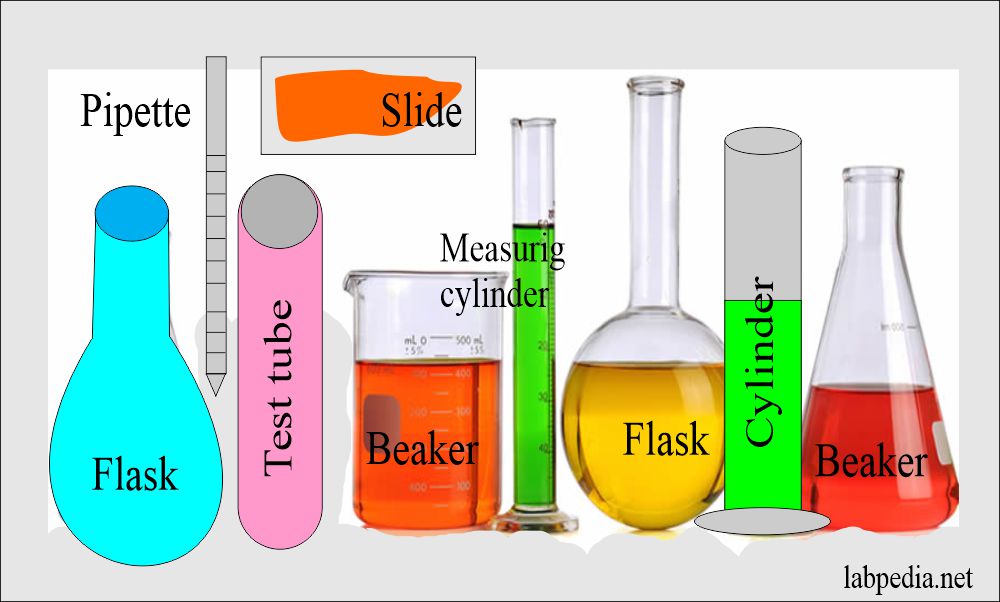
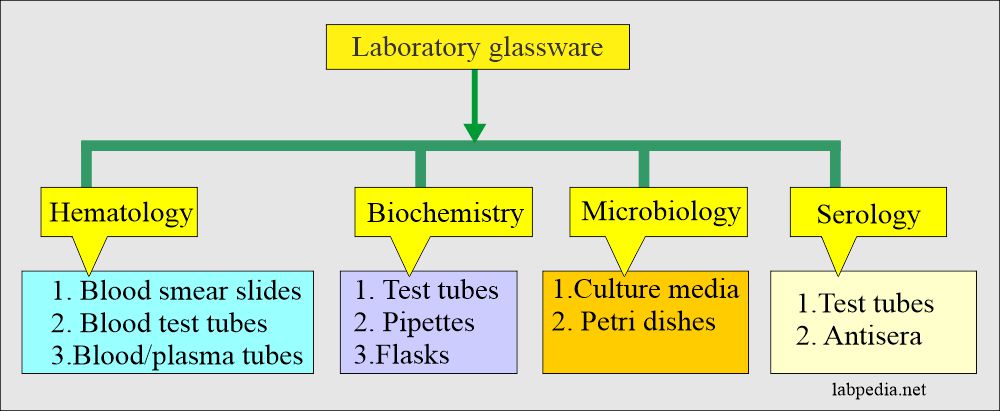
Laboratory Glassware
In any laboratory, the proper cleaning of the glassware is the key to get the correct result.
Definition of Glassware
- Most of the laboratory ware utilized in the clinical chemistry laboratory is either glass or plastic; these may be of different types.
- Glass is a complex silicate, and its properties depend on the type of silicate anions in the structure and its cations.
- Adding metal cations, such as iron (Fe+++) or nickel (Ni), to the glass’s basic structure can alter the color.
- Adding boron oxide (B2O3) can change the thermal properties of glass. This is basically called borosilicate glass and is extensively used in the clinical laboratory.
Properties of the borosilicate glass:
- Resistant to the high temperature.
- It has low alkali contents.
- It is free of the zinc (Zn) group element.
- It is free of heavy metals like arsenic and antimony.
- It can resist heat, corrosion, and thermal shock.
- With heat, its dimension changes very little, so you can use it for heating or sterilization.
Effect of the heat on borosilicate glass:
| The durability of the glass | Temperature |
| Strain point (It is the maximum temperature at which you can use a glass. | 510 °C |
| Annealing point (It is the so-called stress-relief point) | 555 °C |
| Softening point ( It is the temperature at which a glass softens) | 820 °C |
Advantages of the borosilicate glass:
- These are free from Zinc group elements.
- These are free from heavy metals like arsenic and antimony.
- These are resistant to heat and thermal shock.
- There are resistant to corrosion.
- Their size changes very little on heating, so they are preferred when heating or sterilizing heat.
Another type is called soft glass:
- These are boron-free glassware and are also called soft glass.
- Advantages are:
- These have high resistance to alkali.
- These are used for a strongly alkaline solution.
- Their common use is in solutions or digestion involving strong alkalis.
- Disadvantage:
- Its thermal resistance is much less compared to borosilicate glass.
Types of laboratory glassware:
- Pyrex consists of borosilicate.
- Borosilicate glass is free of zinc and heavy metals.
- These resist heat, corrosion, and thermal damage.
- These are very good for heating and sterilization.
- Corex consists of aluminosilicate.
- These are made strong chemically rather than by heat.
- These are 6 times stronger than borosilicate glass.
- These are resistant to alkali and scratching.
- This glass can withstand high heat >250 °C.
- Examples are cylinders and centrifuge tubes.
- Vycor is also called corning brand glass.
- This has high silica and is acid and alkali resistant.
- These can withstand high temperatures (heat shock).
- It can withstand extremes of acids and alkalis.
- These can be heated to 900 °C.
- These can withstand heat shock from 900 C to 0 °C.
- Boron-free glassware has marked resistance to the alkali.
- Its thermal resistance is less than that of borosilicate glass.
- It needs a heating and cooling carefully.
- This is used in chemical reactions where strong alkalis and digestion are involved.
- This is often referred to as soft glass.
- The low actinic glass is amber-colored.
- These are amber or red color glass due to the presence of some chemicals in them.
- Protects the serum from light.
- It is used to handle bilirubin, carotene, and vitamin A. These are sensitive to light when it is in the range of 300 to 500 nm.
- Flint glass has a high index of refraction.
- These are soda-lime glasses consisting of silicon, calcium, and sodium oxide.
- These are the cheapest of all.
- These are not resistant to high temperatures.
- It cannot tolerate heat shock.
- Resistant to the chemical is not strong.
- This glass is easy to melt and shape. These are used to make bottles and some disposable laboratory glassware.
- This glassware must be rinsed with water when used the first time.
- The most important step is to keep the glassware after use in a 3% Lysol (or any other disinfectant) solution for disinfection.
- For suspicion of Tuberculosis, use 1% sodium hypochlorite. This can also kill the HB virus as well.
Options to clean the glassware:
- In most of the big labs, glassware is washed in three steps:
- Automatic washer.
- Followed by a special rinsing cycle.
- Then keep it in the automatic dryer (below 100 °C).
- Or rinse the glassware with a water-miscible organic solvent and then exposing to a stream of air or nitrogen.
- Automatic washer.
- The most commonly used for cleaning are chemicals.
The most common method to clean glassware:
- Decontaminate the glassware by preserving it in 5% bleach or boiling it.
- Can use any detergent or cleaning powder.
- Autoclave may be the alternate method.
- If the glassware is soaked in water after use, that is ideal.
- If leftover, then keeps it in the detergent solution overnight.
- Now rinse with tap water, followed by a rinse with deionized water.
- Can use any detergent or cleaning powder.
- The most common disinfectant are:
- Chlorine-releasing chemicals where chlorine is active against gram-positive and negative bacteria, including HIV and HB viruses.
- Examples are hypochlorite (bleach solution), used in domestic and laundry.
- Aldehydes are formaldehyde and Glutaraldehyde.
- Alcohol used in ethanol or propanol is 70 to 80% V/V.
- Phenols like hycolin, Clearsol, Stericol, and Printol.
- Chlorine-releasing chemicals where chlorine is active against gram-positive and negative bacteria, including HIV and HB viruses.
- In the case of new glassware:
- Boil the glassware in a detergent solution, which will cause the lysis of the organism.
- Cool and again wash thoroughly in tap water followed by the distle water.
- Dry in a hot air oven.
- Sterilize by autoclave at 15 lbs for 20 minutes.
- In the case of handwashing:
- The detergents must be nonionic, metal-free, and not highly alkaline.
- Also, ensure adequate rinsing.
Test to check the cleaning of glassware:
- The best test to see the cleanliness of the glassware is:
- Observe the glass surface as the final rinse water drains off.
- The water should move with a sheeting action, leaving a thin film over the whole surface.
- If the film breaks up into droplets or the surface is unevenly wet, it means the piece is not clean.
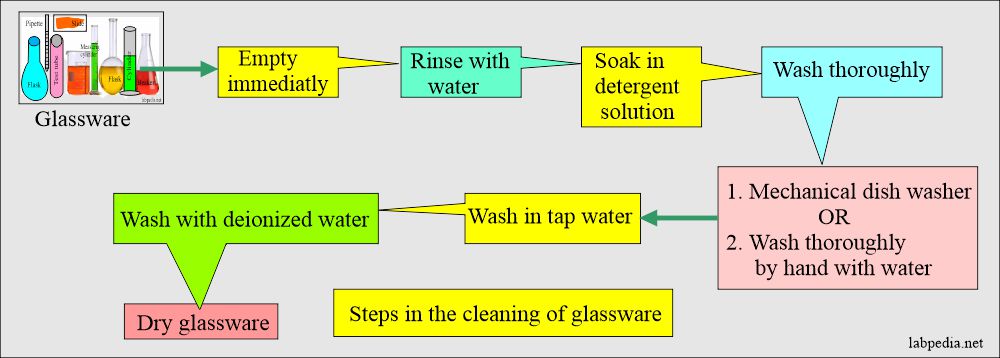
- This process can be made easy if:
- Empty all the glassware after the tests.
- Rinse with water.
- Soak in the solution of detergent.
- For sensitive tests, use disposable test tubes.
We will discuss first the chemical options:
Cleaning Basics steps are:
- It’s generally easier to clean glassware if you do it right away.
- When detergent is used, it can use commercially available as Liquinox or Alconox.
- The detergent should meet the following criteria:
- It can soften the local water supply.
- It should be able to remove organic material at a temperature of 60 °C.
- It should have a neutral pH after rinsing with water.
- Glassware should be free of the microbiological organism after the following rinsing.
- The detergent should meet the following criteria:
- Much of the time, detergent and tap water is neither required nor desirable.
- You can rinse the glassware with the proper solvent.
- Then finish up with a couple of rinses with distilled water.
- A final rinse follows this with deionized water.
Common Lab Chemicals used for Glassware are:
- Water Soluble Solutions, e.g., sodium chloride or sucrose solutions.
- Rinse 3-4 times with deionized water.
- Water Insoluble Solutions. e.g., solutions in hexane or chloroform. Rinse 2-3 times with ethanol or acetone.
- Rinse 3-4 times with deionized water,
- Strong Acids. e.g., concentrated HCl or H2SO4. Wash Under the fume hood.
- Carefully rinse the glassware with copious volumes of tap water.
- Rinse 3-4 times with deionized water. Then let it dry.
- Strong Bases, e.g., 6M NaOH or concentrated NH4OH. Wash Under the fume hood.
- Carefully rinse the glassware with copious volumes of tap water.
- Rinse 3-4 times with deionized water, then let it dry.
- Weak Acids, e.g., acetic acid solutions or dilutions of strong acids such as 0.1M or 1M HCl or H2SO4.
- Rinse 3-4 times with deionized water and then let it dry.
- Weak Bases, e.g., 0.1M and 1M NaOH and NH4OH. Rinse thoroughly with tap water to remove the base.
- Then rinse 3-4 times with deionized water and let it dry.
Washing Special Glassware used for Organic Chemistry:
- Rinse the glassware with the appropriate solvent.
- Use deionized water for water-soluble contents.
- Use ethanol for ethanol-soluble contents, followed by rinses in deionized water.
- Rinse with other solvents as needed, followed by ethanol and deionized water.
- If the glassware requires scrubbing, scrub with a brush using hot soapy water, rinse thoroughly with tap water, followed by rinse with deionized water
Washing with soap and water:
- Soak the glassware in soap solution for at least 10 to 15 minutes or leave overnight.
- Scrub with a brush or cloth or sponge if needed.
- Rinse thoroughly with tap water.
- Again rinse with distilled or deionized water.
- If you need this glassware soon, rinse it with acetone or ethanol.
Burets (Cleaning)
- Wash with hot soapy water, and rinse thoroughly with tap water.
- Then rinse 3-4 times with deionized water.
- Burets need to be thoroughly clean to be used for quantitative lab work.
Pipettes and Volumetric Flasks (cleaning)
- May need to soak the glassware overnight in soapy water.
- Clean the pipette and volumetric flasks using warm soapy water.
- The glassware may require scrubbing with a brush.
- Rinse with tap water, followed by 3 – 4 rinses with deionized water.
Drying Glassware
- If glassware is to be used immediately after washing and must be dry.
- Rinse it 2-3 times with acetone. This will remove any water and will evaporate quickly.
- This is not a great idea to blow air into glassware to dry it.
- Acetone may be used for a final rinse of sensitive or urgently needed glassware. The solvent is miscible with water and helps dilute and wash away the remaining water from the glassware.
Wash labware as quickly as possible after use:
- If a thorough cleaning is not possible immediately, put glassware to soak in water.
- If labware is not cleaned immediately, it may become impossible to remove the residue.
- Put into acid water (a 1% solution of hydrochloric or nitric acid) before washing. Can keep for several hours in this solution.
- Brushes with wooden or plastic handles are recommended as they will not scratch or abrade the glass surface.
Chromic acid used for dirty glassware:
- If glassware becomes unduly clouded or dirty or contains coagulated organic matter, it must be cleaned with chromic acid.
- When the chromic acid solution is used, the item may be rinsed with the cleaning solution or filled and allowed to stand.
- Time depends on the amount of contamination on the glassware. Relatively clean glassware may require only a few minutes.
- If more contaminated, then keep it overnight.
- Special types of precipitates may require removal with nitric acid or sulfuric acid. No doubt, these are very corrosive.
Hematological glassware needs special precautions:
- Do not use detergents because if there is a minute concentration, that may lead to RBCs’ hemolysis.
- So for general Tubes, pipettes, and slides, wash these thoroughly under tap water. Can use a brush to remove any leftover from the glassware.
- Keep the hematology-used material in a dichromate solution for 12 to 24 hours. Then again, wash thoroughly with tap water.
- Allow draining.
- Dry in the hot oven.
Blood pipettes need the following method to clean:
- With the help of a suction pump or handheld suction pump, draw tap water through these pipettes.
- Use distilled water for suction and washing.
- Last, can use acetone for the same purpose.
- Let them dry in the air.
- If there are blood microclots, keep them in 10% potassium hydroxide for 12 to 24 hours.
Conclusion and Important facts:
- Should clean the glassware as soon as possible.
- In the case of delay, put the glassware in water.
- In the case of late cleaning, the residue may not be removed.
- New, slightly alkaline glassware needs to be soaked in acid water (1% HCl or HNO3) for several hours before washing.
Cleaning solution to prepare in the lab:
- Sodium dichromate = 20 grams (technical grade)
- H2SO4 (concentrated, technical grade) = 300 mL.
- Procedure:
- Mix sodium dichromate in water to make a thick paste.
- Add carefully H2SO4 in a small quantity with constant stirring.
- Take the clear upper solution for use.
- Can use it till there is a reddish color to the dichromate.
- Discard when it changes from reddish to green color.
- This solution can remove organic and as well as inorganic material because of its strong oxidizing power.
- Should not use this solution on plasticware except Teflon.
- After sodium dichromate cleaning, thoroughly rinse with tap water and then reagent water.
- Precautions:
- This is a powerful corrosive reagent, so you must wear rubber gloves, safety glasses, and a rubber or plastic apron.
Nitric acid (HNO3)
- Strong nitric acid is used, which is better to use in the hood.
Hydrochloric acid (HCL)
- This is used as I mol/L or less.
- This will not need a hood.
Drying method
Various methods like:
- Air drying.
- Oven at a temperature below 100 °C and the laboratory ware kept bottom up.
- Sometimes, the glassware is rinsed with a water-miscible organic solvent and then exposed to a stream of air or nitrogen.
- Store the glassware and protect it from dust.
Hazards and safety of the glassware
- The main hazard is an injury while handling this damaged glassware.
- Avoid disposable glassware because it is thin-walled and easily breakable. These are not for cleaning and reuse.
- Buy cover glass No. 2 because these are thicker and easily not damaged.
- Check before use for any crakes or broken and chipped ends. These may cause injury while handling. It is better to discard all this glassware.
- Never centrifuge-damaged glassware.
- When handling glassware, wear protective gloves.
- Discard the broken glassware in special bags marked with sharp.
- When there is broken glass on the floor, immediately clean the floor.
Plastic labware (Plasticwares)
- Whenever possible, use plasticware instead of glassware.
- Plasticware is now available as beakers, graduated cylinders, bottles, funnels, centrifuge tubes, pipettes, and tubing.
- Advantage:
- Plasticware is unbreakable.
- It does not release ions like glassware.
- There is high corrosion resistance.
- Disadvantage:
- It tends to bind various solutes and leads to the surface-bound material in the subsequent solution.
- Polyethylene is permeable to water vapor, even in tightly stoppered bottles. This will leads to the concentration of the reagents.
- Polyethylene is not inert and may bind or absorbs proteins, dyes, iodine, stains, and picric acid.
- Advantage:
Types of plasticware:
Polyethylene:
- These are used to make bottles, beakers, jars, jugs, funnels, pipet jars, tanks, buret covers, check valves, needle valves, hollow stoppers, etc.
- Advantages:
- These are cheaper than polypropylene and are used in most disposable plasticware.
- Disadvantages:
- It is permeable to water vapors. This will leads to the concentration of the reagents and calibrators.
- It is not completely inert, and it can absorb proteins, dyes, stains, iodine, and picric acid.
Polyolefins:
- Advantages:
- Concentrated H2SO4 (sulphuric acid) does slowly attack it at room temperature.
- As a group, these are unaffected by the acids, alkalis, salts solutions, and most of the aqueous solution.
- Disadvantages:
- These are chemically relatively inert.
- Aromatic aliphatic and chlorinated hydrocarbons cause moderate swelling at room temperature.
- Organic acids, essential oils, and halogens slowly penetrate these plastic wares.
Polypropylene:
- Advantage:
- It can withstand high temperatures.
- It can be sterilized.
- Disadvantage:
- It absorbs pigments and tends to become discolored.
Polycarbonate:
- These are also used to make various laboratory utensils.
- Advantages;
- These are twice strong as compared to polypropylene.
- These can be used by heating with the range of -100 °C to +160 °C.
- These are used extensively in centrifuge tubes and graduated cylinders.
- Disadvantages:
- These are unsuitable to use with strong acids, bases, or oxidizing agents.
- Chlorinated aliphatic and aromatic hydrocarbons will dissolve it.
- It is insoluble in aliphatic hydrocarbons, some alcohols, and dilute aqueous acids and salts.
- These are unsuitable to use with strong acids, bases, or oxidizing agents.
Fluorocarbon resin (plastic, Teflon):
- These are the unique plastic wares that make these chemically inert.
- Advantages:
- These are resistant to corrosion at high temperatures.
- Teflon resists extreme temperatures ranging from -270 °C to +255 °C.
- Bottles and beakers made of this material are suitable for use in cryogenic experiments.
- These are translucent and white.
- These are inert to corrosive reagents as boiling HNO3 or H2SO4 acids.
- These will also tolerate boiling hydrocarbons, ketones, esters, and alcohols.
- Teflon is used for self-lubricating stopcock, stirring bars, bottle cap liners, and tubing.
- It is quite easy to clean and fast drying.
- Disadvantage:
- It can easily be scratched.
- It can easily be bent.
Physical properties of the various types of plastic wares:
| Type of the plastic | Maximum tolerable temprature | Possibility of autoclave | Transparency |
| Low-density polyethylene | 80 °C | Not possible | Translucent |
| High-density polyethylene | 120 °C | Not possible | Translucent |
| Polypropylene | 135 °C | Possible | Translucent |
| Teflon (fluorinated ethylene propylene) | 205 °C | Possible | Translucent |
| Tefzel (Ethylene-tetra-fluoroethylene) | 150 °C | Possible | Translucent |
| Polycarbonate | 135 °C | Possible | Clear |
| Polyvinyl chloride | 70 °C | Not possible | Clear |
Cleaning of the plasticware:
Whenever possible, use plastic laboratory ware.
- Advantages are:
- These are nonbreakable.
- Do not release ions into the solution like glass.
- This plasticware can be cleaned in the washing machines.
- Ultrasonic cleaners can be used when you keep this plasticware on the transducer diaphragm.
- Polypropylene, Teflon, and polymethyl pentene may be repeatedly autoclaved under normal conditions.
- Polycarbonate should be autoclaved at 121 °C for 20 minutes.
- Disadvantages are:
- These tend to bind various solutes.
- Polyethylene is permeable to water vapors.
- This evaporation leads to the concentration of the reagents and standards.
- So don’t keep a small amount of the reagent in oversize plastic tubes.
- Polyethelene is not completely inert, so it can bind or adsorb proteins, dyes, stains, iodine, and picric acid.
- Avoid doing the creatinine test in plastic test tubes; use glass tubes.
- Colorless reagents may bind to the plastic ware without being detected.
- It is observed that there is a slow reduction of the ceric and cuprous ions in polyethylene bottles.
- Should avoid the use of abrasive cleaners and strong oxidizing agents.
Procedure to Clean the plasticware:
- Plasticware should be well cleaned and then rinsed with deionized water before sterilization.
- The above cleaning is essential because some chemicals have a bad effect on autoclave temperature.
- Polystyrene, polyvinyl chloride, styrene-acrylonitrile, and polyethylene are not autoclavable, so these may be sterilized with gas (ethylene oxide).
- The above plasticware can also be sterilized by rinsing it with benzalkonium chloride.
- Except for the Teflon, no other plasticware should be sterilized hot-air because of oxidative degradation.
- Plasticware may be dried in an oven at 110 °C.
Sterilization
- The following methods can do sterilization:
- Dry Heat:
- Sterilization has limited value. Prolonged exposure may cause damage.
- Hot air oven:
- Where heat is transferred by convection, conduction, or radiation.
- A temperature of 100 °C for one hour can destroy the nonsporing organism. Fungal spores need 115 °C for one hour.
- While other bacteria at 160 °C temperature is needed for one hour.
- Incineration:
- Where the flame is an effective way of sterilization. Flame heat is needed for the loop for culture.
- Moist heat:
- It is the most reliable method of sterilization. This is the most lethal agent to kill microorganisms.
- Microbial death is due to coagulation and denaturation of the protein and enzyme.
- Boiling:
- It is not effective in killing spore-bearing bacteria and for surgical instruments.
- Steam sterilization:
- Steam sterilization or Tyndallization is exposure to steam at 100 °C for 90 minutes. This good means to sterile the media which contain sugar.
- The autoclave:
- It heats water under pressure, which boils at progressively higher temperatures. This method is good for rubber materials and surgical instruments.
- Membrane filters:
- These are Millipore filters. Filters with a pore of 0.22 micrometers are sufficient for the bacteria.
- Seitz filter:
- It is a disposable asbestos pad filter.
- Flaming:
- It is when the material is wetted by alcohol and then flamed. This method is rapid.
- Ultraviolet light:
- It causes damage to bacteria.
- Radiation:
- Radiation in the form of beta and gamma X Rays is used for surgical pads.
- Supersonic and ultrasonic:
- These waves, 9000 cycles per second or above, are used to rupture and disintegrate the cells.
Questions and answers:
Question 1: Is there any complication of dry heat?
Question 2: What are advantages of the plasticware in comparison to glassware?

Excellent ,I learn more things from your’s.
Thanks
Thanks for the remarks.
Thank you very much, I really appreciate your efforts.
My Regards
Thanks a lot.
Thank you for your contribution, I’m really appreciate thank you very much
Thanks
Hello! I loved this article and was wondering if you have best practices for using Potash (Potassium Hydroxide) Food Grade to clean/sterilize glassware
I really don’t know, and I think it is safe for foods, but it may not clean the contaminated laboratory glassware.
Hello Sir, can we wash the 50ml centrifuge tubes acetone. If it is possible what is the procedure to clean the tubes with acetone.
Please see this reference:
ACETONE. … Acetone is an excellent way to remove organic residues on your glassware, and it is used like “water” by organic chemists. It can also help dry your newly washed glassware after it has been rinsed with DI water if you plan on using it in the same lab period and don’t have time to wait for your DI water to dry.
Another reference:
Acetone is used for the common cleaning of laboratory wares for a few reasons. Firstly it’s because Acetone is a very good solvent, it is a very polar substance that dissolves almost all organic compounds, which is obviously critical if you’re cleaning. It is water-miscible, so can be used in conjunction with water.
Can glassware containing formaldehyde be cleaned such that it would be considered safe for foods?
These methods are for laboratory glassware. I don’t think we can use it for foods.
why do you need to clean all moisture content on a glassware before putting it in the hot oven air for sterilization?
If you don’t remove moisture, it will leave spots on the glassware.
We have every kind of glassware in our lab and the methods you mentioned are already in use. All I was wondering about was the thing whether there is any liquid in the market for cleaning lab glassware or a spray wipe which will make work easier?
I am sending you some links”
https://www.fishersci.com/shop/products/fisherbrand-losuds-liquid-glassware-detergent/13641730?ef_id=Cj0KCQjwhsmaBhCvARIsAIbEbH5JXUKeoQlJhrssUA-P6Qo4G51i_NyAFXwa3A6kAkGJ11mrYe0dERsaAhv1EALw_wcB:G:s&ppc_id=PLA_goog_16713066638__13641730___7624972691594846525&ev_chn=shop&s_kwcid=AL!4428!3!!!!x!!13641730&gclid=Cj0KCQjwhsmaBhCvARIsAIbEbH5JXUKeoQlJhrssUA-P6Qo4G51i_NyAFXwa3A6kAkGJ11mrYe0dERsaAhv1EALw_wcB
https://www.fishersci.com/us/en/products/I9C8L9O4/labware-detergents-cleaners.html
You can also see options on Amazon.com.
Dear Dr. Riaz, Can you advise on the Class A glassware cleaning? What is the maximum temperature at which it can dry? Do you have any regulatory or manufacturer evidence to justify the same?
Please see this link:
https://www.labmanager.com/lab-health-and-safety/how-to-clean-laboratory-glassware-20325
I really appreciate about such nice information.
Thanks for the comments.
How long can autoclaved glassware last in an open, cool temperature of 21°c?
I am sending you a few references from a google search:
1. Supplies wrapped in double-thickness muslin comprising four or equivalent layers remain sterile for at least 30 days. Any item that has been sterilized should not be used after the expiration date has been exceeded or if the sterilized package is wet, torn, or punctured.
2. Conclusion: For small metal instruments, autoclaved packages in double-wrapped linen or double-wrapped plastic-paper combinations can be stored safely for at least 96 weeks.