Klebs loeffler bacilli (KLB), Corynebacterium Diphtheriae, Diagnosis and Stain,
Corynebacterium Diphtheriae
Sample for Corynebacterium Diphtheriae
- The swab is made from the throat or from the suspected lesion.
Indication for Corynebacterium Diphtheriae
- For the diagnosis of C. Diphtheriae (Diphtheria)
Microbiology of Corynebacterium Diphtheriae
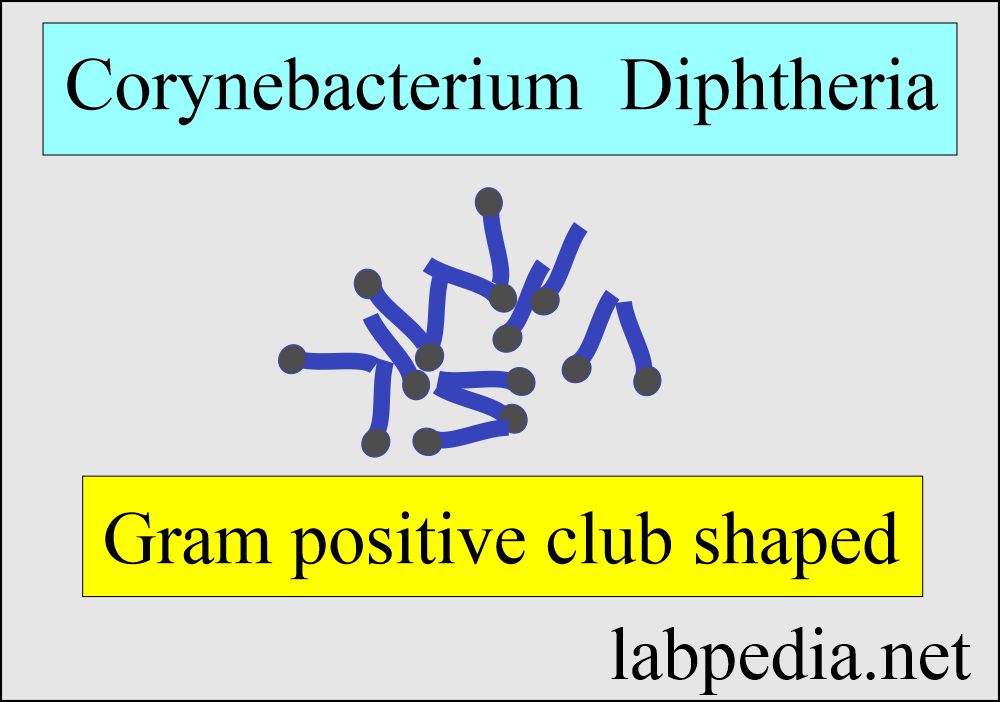
- C. diphtheriae is a nonmotile, non-capsulated, club-shaped, Gram-positive bacillus.
- These are non-sporing.
- Highly pleomorphic-like Chinese letter appearance.
- Habitat is the throat and nose of human beings.
- These are pleomorphic gram-positive rods or clubs which divide in unique patterns like V, L, or W shapes, the so-called Chinese character shapes.
- These rods measure 3 x 0.3 µm.
- Culture:
- These are aerobes and facultative anaerobes.
- The optimum temperature for the growth is 37 °C.
- Media containing blood or serum is needed.
- Selective media is needed for isolation.
- Most strains require nicotinic and pantothenic acids for growth; some also require thiamine, biotin, or pimelic acid.
- The selective medium is used:
- Loeffler’s medium (coagulated blood serum.
- Take the growth after 12 hours and stain the growth with methylene blue, showing rod-shaped pleomorphic bacteria.
- Potassium tellurite medium.
- The colonies are black to gray within 24 hours of the culture.
- Loeffler’s medium (coagulated blood serum.
- The medium should be supplemented with amino acids for optimal production of diphtheria toxin.
- Biochemical reactions:
- There is acid production from carbohydrates.
- Gravis strain can ferment starch and sugar.
- Intermedius and mitis can not ferment.
- Toxigenic strains are lysogenic.
- These toxigenic strains cause the death of the experimental animal in 2 to 3 days,
- While antitoxin-protected animals can survive.
- Corynebacterium diphtheriae subtypes are based on colony morphology:
- Mitis.
- intermedius.
- Gravis.
- For epidemiological studies, the C. diphtheriae typing is done by:
- Serotyping.
- Phage typing.
- Bacteriocin typing.
| C. diphtheriae | Growth on Loeffler’medium | Growth (colony) on Tellurite medium |
| C. Mitis | There are numerous granules and typical arrangement | Colonies are of medium size, circular and convex. These are glistening and black |
| C. Intermedium | There are short, irregularly staining rods without metachromatic granules. The arrangement is like Chinese letters. | There are small and smooth colonies with irregular edges. These are grey-black with a pale periphery. |
| C.Gravis | These are club-shaped and have few metachromatic granules. | Colonies are flat gray with a raised center. Edges are irregular, and there is a daisy-head appearance. |
Pathogenesis of Corynebacterium Diphtheriae:
- The asymptomatic nasopharyngeal carriage is common in regions where diphtheria is endemic.
- In susceptible individuals, toxigenic strains cause disease by multiplying and secreting diphtheria toxin in either the nasopharyngeal area or skin lesions.
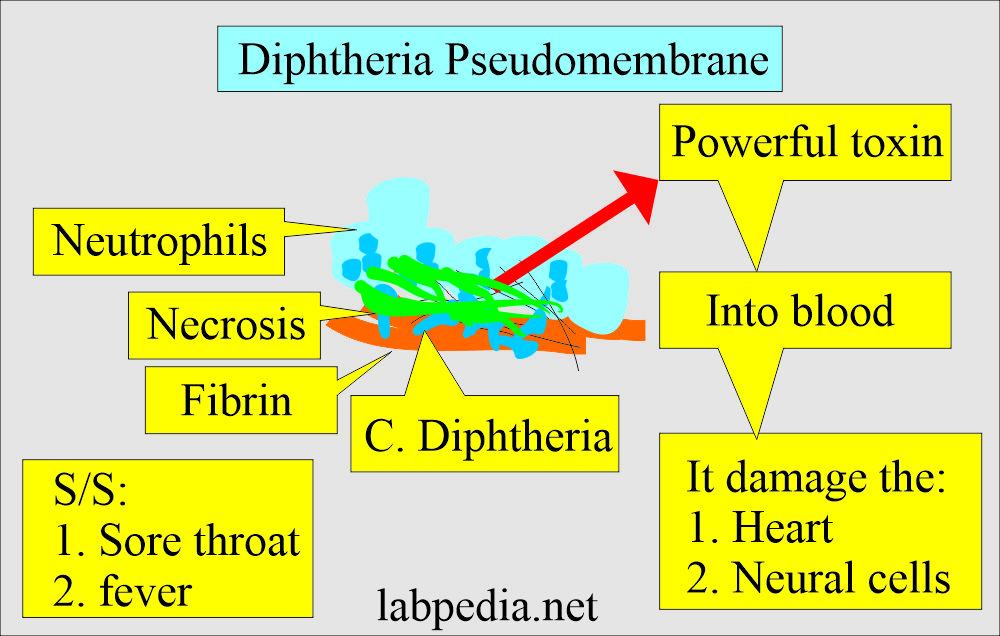
- There is often the formation of a pseudomembrane composed of fibrin, bacteria, and inflammatory cells.
- The necrosis of the affected epithelium forms a pseudomembrane of fibrin, necrotic cells, and neutrophils.
- After absorption into the blood, the toxin acts systemically on the myocardium, nervous tissue, and adrenal gland.
- Only the motor nerves are affected.
- Diphtheria toxin can be proteolytically cleaved into two fragments:
- Fragment A catalyzes the NAD+-dependent ADP-ribosylation.
- Fragment B binds to the cell surface receptor and facilitates the delivery of fragment A to the cytosol.
- The toxin can be inactivated by treating it with formaldehyde, and there is the formation of toxoids.
- Toxoid is used for immunization.
Clinical presentation of Corynebacterium Diphtheriae (Signs and symptoms):
- C. diphtheriae infects the nasopharynx or skin.
- Toxigenic strains secrete a potent exotoxin which may cause diphtheria.
- The symptoms of diphtheria include:
- Pharyngitis.
- Fever.
- Swelling of the neck or area surrounding the skin lesion.
- Diphtheritic lesions:
- These are covered by a pseudomembrane.
- The toxic outcome is that toxin is distributed to distant organs by the circulatory system and may cause:
- Paralysis.
- Congestive heart failure.
- These are the clinical signs of nasopharyngeal diphtheria infection:
- A sore throat.
- Dysphagia.
- Bloody nasal discharge.
- Pseudomembrane formation.
Diagnosis of Corynebacterium Diphtheriae:
- Clinical diagnosis depends upon culture-proven toxigenic C. diphtheriae infection of :
- The skin.
- Nose or throat.
- Toxigenicity is identified by a variety of in vitro (e.g., gel immunodiffusion, tissue culture) or in vivo (e.g., rabbit skin test, guinea pig challenge).
- This bacteria is Urease negative.
- Schick test:
- This is a skin test to find immunity for circulating diphtheria antitoxin.
- This test indicates either previous immunization or infection.
- Inject intradermally on an anterior aspect of the forearm toxin.
- Also, inject a heat-inactivated toxin into another arm.
- Result: The positive test is erythema at the site of injection.
- Negative erythema on the heat-inactivated arm.
- The arm should be examined after 1 to 2 days.
- Again examine after 5 to 7 days.
- Special stain:
- Albert’s stain.
- Another stain used is Ponder’s stain.
Staining procedure:
- Cover the fixed and dried slide with Loeffler’s methylene blue. Leave for 5 minutes.
- Wash with water.
- Decolorize with sulphuric acid with a dilution of 1/1000 immediately.
- Acid should not stay for more than a few seconds.
- Again wash with water.
- Treat with Gram’s iodine.
- Again wash with water.
- Counterstain with 1% eosin for 30 seconds.
- Wash with water and dry it.
Result of staining:
- Metachromatic granules are seen on the smear.
- The body of the bacteria is pinkish, and the granules are blue-black.
- Gram stain also shows gram-positive bacteria.
Treatment Corynebacterium Diphtheriae:
- Give supportive treatment.
- Give antitoxin, which will neutralize the diphtheria toxins. This will be effective in the early stages when the toxins have not reached the target organs.
- Antibiotics like penicillin or erythromycin will kill the bacteria. It will make patients noncontagious.
- The vaccine should be given because the patient doesn’t develop immunity to future infection.
- DPT (Diphtheria, pertussis, and tetanus) vaccine is given.
Questions and answers:
Question 1: What are effects of C. Diphtheria toxin.
Question 2: What type of vaccine is used for Diphtheria infection.

Very good, nice lecture. Thanks