Enzymes:- Part 2 – Acid phosphatase, Alkaline Phosphatase, Amylase and Angiotensin-Converting Enzyme
Diagnostic Value of Various Enzymes
- These enzymes have clinical significance for the diagnosis of various diseases.
Acid phosphatase (ACP)
What is the best sample for acid phosphatase?
- Blood was collected to prepare the serum.
- Acidification of the serum to a pH below 6.5 will stabilize the enzyme.
- Avoid lipemic serum, which interferes with the test.
How will you define the acid phosphatase enzyme?
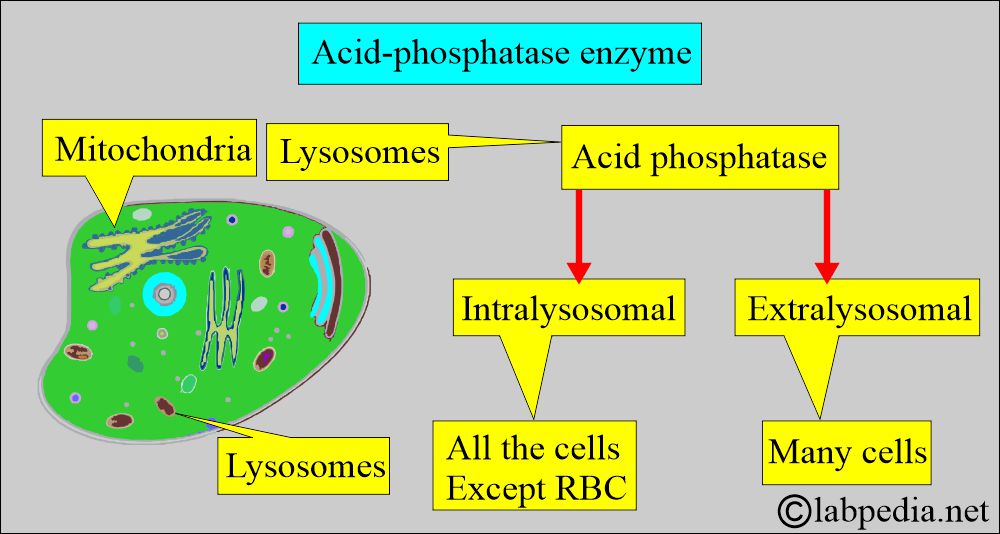
- Acid phosphatase is a hydrolytic enzyme secreted by various cells.
- Prostatic tissue is rich in acid phosphatase enzyme. Carcinoma originating from the prostatic tissue will have the ability to produce acid phosphatase enzyme.
- Acid phosphatase has optimal activity below pH 7.0.
- Prostatic ACP shows optimal activity at pH 5-6.
- The ACPs are unstable at temperatures above 37 °C and above 7.0.
What is the distribution of the acid phosphatase enzyme?
- Acid phosphatase (ACP) is present in the lysosomes, except in the RBCs. Extralysosomal ACP is also present in many cells.
- Acid phosphatase has six isoenzymes.
- Prostatic acid phosphatase (PAP).
- Red cell acid phosphatase.
- Platelets acid phosphatase.
- Bone acid phosphatase.
- Liver acid phosphatase.
- leucocyte acid phosphatase.
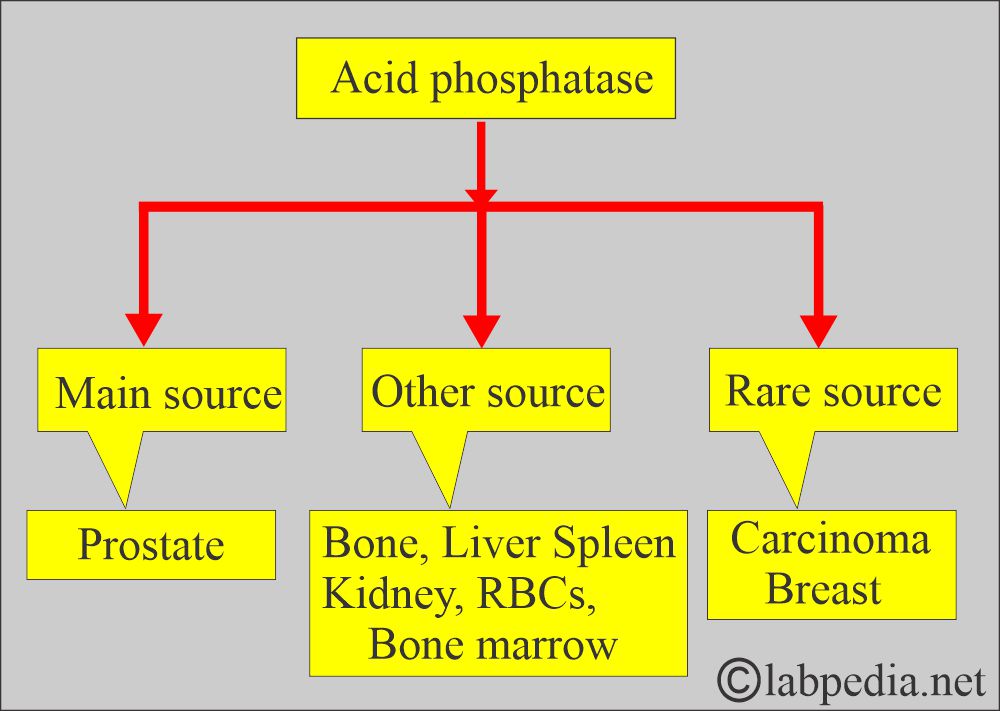
- The greatest concentration of ACP activity occurs in the liver, spleen, milk, RBCs, bone marrow, platelets, and prostate glands.
- The prostate is the richest source of ACP. It is also found in semen.
- The osteoclast is the source of raised ACP in growing children compared with adults.
What is the clinical significance of Acid phosphatase?
- Osteoclasts are the source of ACP in growing children compared to adults.
- This is used to diagnose or monitor prostatic carcinoma.
- Now, this is not used for the detection of prostate carcinoma.
- A slight or moderate increase in the ACP is seen in Paget’s disease.
- In hyperparathyroidism with skeletal involvement, ACP is raised.
- It is raised in the case of tumor infiltration of the bone, such as breast cancer.
What are the causes of raised acid phosphatase (ACP)?
- Osteoclastoma (Giant-cell tumor).
- Osteoclastic tumors.
- Osteopetrosis (marble bone disease).
- Hairy-cell leukemia also shows osteoclastic type ACP.
What is the Medicolegal importance of acid phosphatase?
- There is a very high concentration of ACP in the semen, which is used in case of a rape investigation.
How do we take samples from the victim of rape?
Method 1:
- Take a swab from the alleged case of a rape victim, and preserve it in 2.5 mL of broth.
- Broth contains one liter of the solution:
- Bovine albumin = 50 grams.
- Sodium azide = 0.2 gram.
- Phosphate buffer at pH 7.4 (phosphate buffer 10 mmol + 9 gram NaCl).
- Store the sample in the broth at 4 °C or room temperature.
- The specimen can retain ACP activity for up to one month.
- Broth contains one liter of the solution:
- Interpretation of vaginal sample:
- Vaginal ACP in noncoital women is <10 U/L (broth sample).
- Post-coital women ACP is >50 U/L.
- Post-coital vaginal ACP takes 4 days to become a non-coital level. So, the sample can be taken during this time.
Method 2:
- Take a vaginal swab and keep it in 1 mL of isotonic saline.
- Freeze the swab.
- Before the test, thaw the swab at 2 to 4°C for 24 hours before the assay.
How will you diagnose prostatic carcinoma?
- ACP is now rarely used for the diagnosis of carcinoma of the prostate.
- The following tests replace ACP:
- Digital rectal examination.
- Transurethral ultrasonic images.
- Histopathological examination of the prostatic biopsy.
- Advise total body scan.
- Advise prostatic specific antigen (PSA).
Alkaline phosphatase (ALP)
What sample will you take for Alkaline phosphatase?
- It is done on the patient’s serum.
- A fasting sample is a better choice.
- This test can also be done on a random sample.
- How to get a good serum sample: Take 3 to 5 ml of blood in a disposable syringe or a Vacutainer. Keep the syringe for 15 to 30 minutes at 37 °C and then centrifuge for 2 to 4 minutes to get the clear serum.
- Keep the sample refrigerated as soon as you separate the serum.
- Serum stored at 0 to 4 °C is stable for 2 to 3 days, and at -25 °C for 1 month.
- Perform the test as soon as possible because ALP activity increases by 3% to 10% when standing at 25° C or 4 ° C for several hours.
What precautions will you take for Alkaline phosphatase?
- Storage at room temperature increases the ALP activity.
- Avoid EDTA and oxalate anticoagulants, which decrease Alkaline phosphatase activity.
- If serum is left at room temperature:
- Then there is a 1% increase in 6 hours.
- 3% to 6% in 1 to 4 days.
- We may even see an increase if the serum is refrigerated; you will see a 2%/day increase.
- Recent food intake may increase the value.
- Values may be 25% higher after taking the high-fat meal.
- Drugs such as allopurinol, antibiotics, colchicine, indomethacin, fluorides, isoniazid (INH), methotrexate, nicotinic acid, methyldopa, phenothiazines, and probenecid can increase alkaline phosphatase levels.
- Drugs such as arsenic, cyanides, nitrofurantoin, and zinc salts may decrease alkaline phosphatase levels.
- Hemolysis may cause a slight increase in the ALP. ALP is 6 times higher in the RBC than in the serum.
What are the indications for Alkaline phosphatase?
- It is advised to detect and monitor liver disease.
- It is advised to diagnose and monitor bone diseases.
What is the distribution of the Alkaline phosphatase?
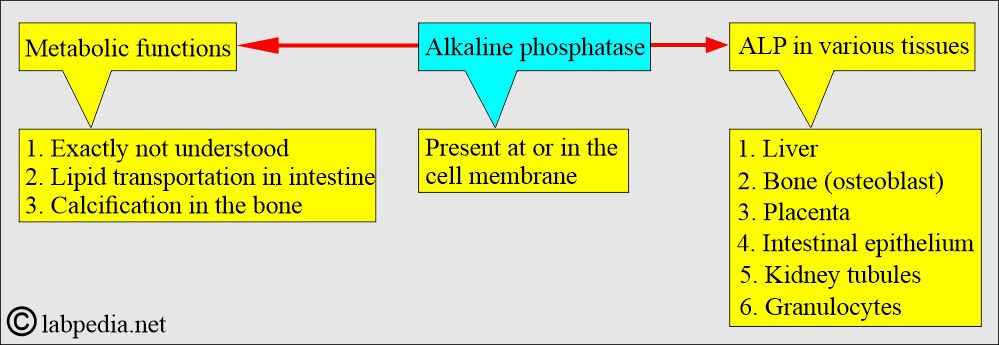
- ALP is found in many tissues, at or in the cell membrane.
- ALP is a nonspecific enzyme that can react with many different substrates.
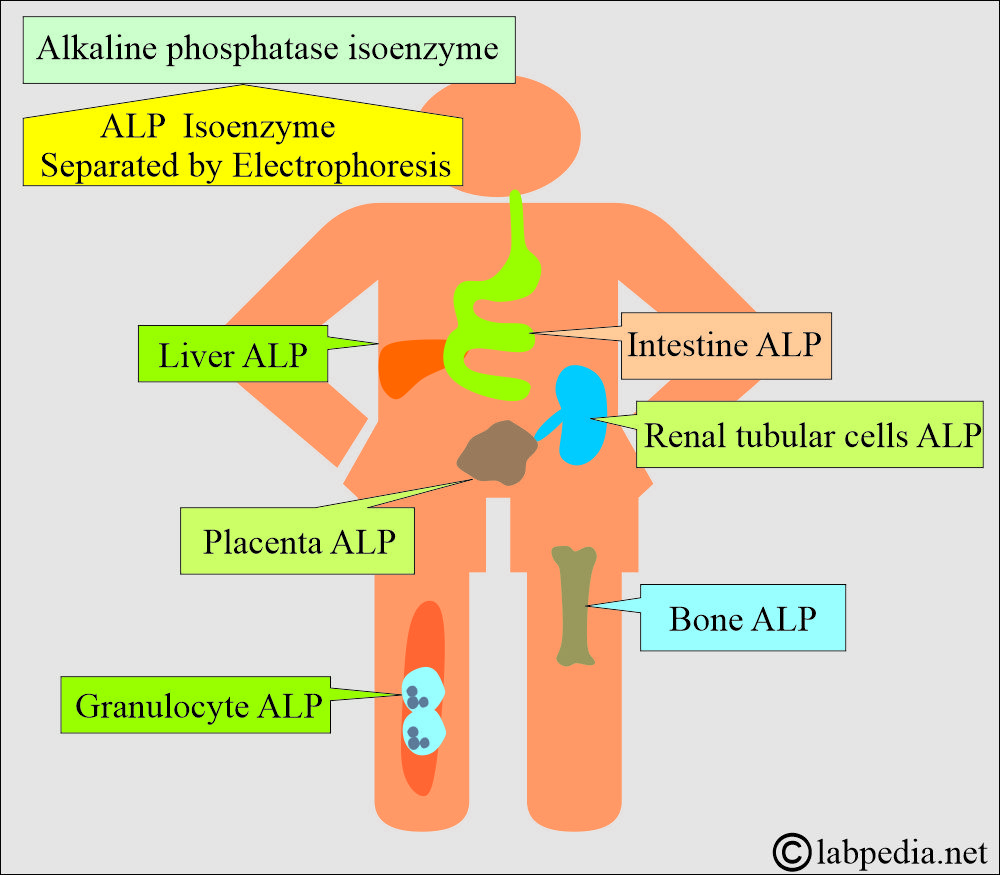
- The highest concentration is found in the liver. Within the liver, ALP is present in the Kupffer cells, which line the biliary collecting system. From there, this enzyme is excreted in the bile.
- ALP is mainly from the liver and biliary tree, nearly up to 50% from the skeleton (osteoblastic cells), a small amount from the intestinal epithelium and renal tubule cells, and a lower concentration in leucocytes and placenta.
- ALP is also found in the intestinal epithelium and the placenta.
What are the properties of Alkaline phosphatase (ALP)?
- This ALP is age-dependent.
- ALP (serum) is denatured at 56 °C and stable at lower temperatures.
- The liver isoenzyme is moderately heated and stable at 55 °C, but the bone isoenzyme is heat-labile.
- The placental isoenzyme is stable at 65 °C, but the others are not.
- Alkaline phosphatase is called alkaline because its function is seen between a pH of 9 and 10, and is best at a pH of 9.0.
- ALP enzymes require Magnesium for the reaction.
- The enzyme’s functions in an alkaline medium are better.
- This is raised in growing children due to osteoblastic activity.
- This enzyme helps to diagnose obstructive jaundice.
- Alkaline phosphatase has different isoenzymes separated by electrophoresis.
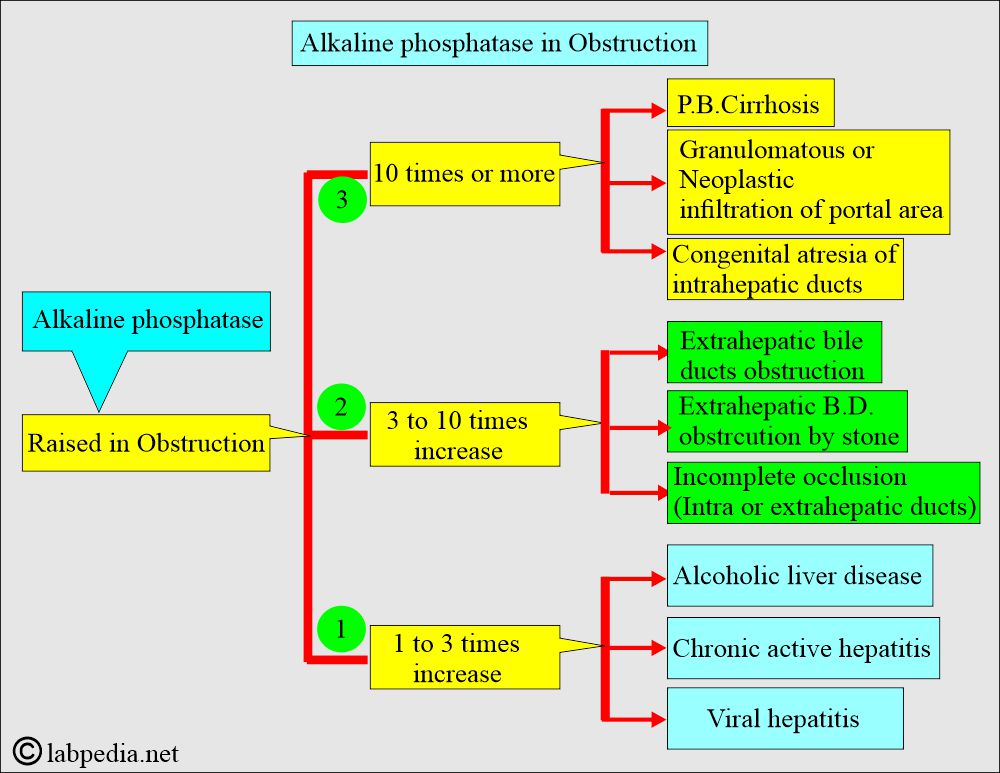
What are the causes of raised Alkaline phosphatase?
- It is raised in bone-associated diseases with increased osteoblastic activity.
- Obstructive jaundice may be raised from 10 to 20 times the normal level.
- This will differentiate hepatocellular disease from cholestatic disease.
Amylase
What sample is needed for amylase?
- This test is performed on the patient’s serum.
- The serum is stable at room temperature for 7 days and at 4 °C for one month.
- Take 3 to 5 ml of blood in the disposable syringe. Keep the syringe for 15 to 30 minutes and then centrifuge for 2 to 4 minutes. In this way, you can get a clear serum.
What Precautions will you take for Amylase?
- Avoid alcohol intake before sampling because it gives rise to an increase in the amylase level.
- Urine can be collected in a 2-hour or a 24-hour sample. Refrigerate the urine.
- Avoid contamination with saliva.
- Lipemia, anticoagulant EDTA, fluoride, and citrate decreased amylase levels.
What are the indications for Amylase?
- To diagnose acute pancreatitis and monitor the treatment.
- To differentiate from other abdominal pain, epigastric discomfort, nausea, and vomiting.
- In cases of ascites, it may be performed to rule out pancreatitis.
How will you define Amylase?
- Amylase in serum comprises pancreatic and salivary isoenzymes, and various methodologies can differentiate them.
- Nonpancreatic causes of the raised amylase level are salivary glands. In renal insufficiency, both types (salivary and pancreatic) are elevated.
How will you describe the pathophysiology of amylase?
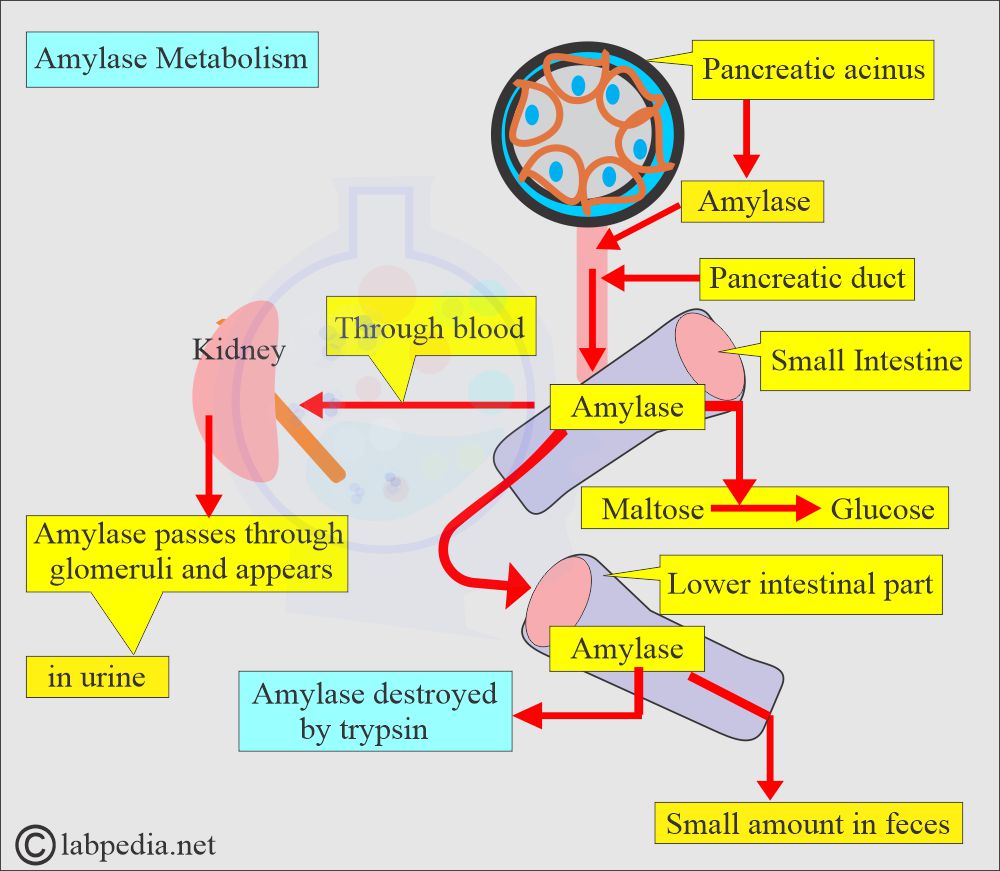
- Amylase is present in many organs and tissues.
- Amylase has a molecular weight of 55,000 to 60,000; this small molecule can pass through the kidneys’ glomeruli and is normally found in the urine.
- It is of two types:
- α-amylase is present in human tissue.
- β-amylase is present in plants and bacteria.
- The greatest amylase concentration is in the pancreas.
- This enzyme is synthesized by the acinar cells and then secreted via the pancreatic duct system into the intestinal tract.
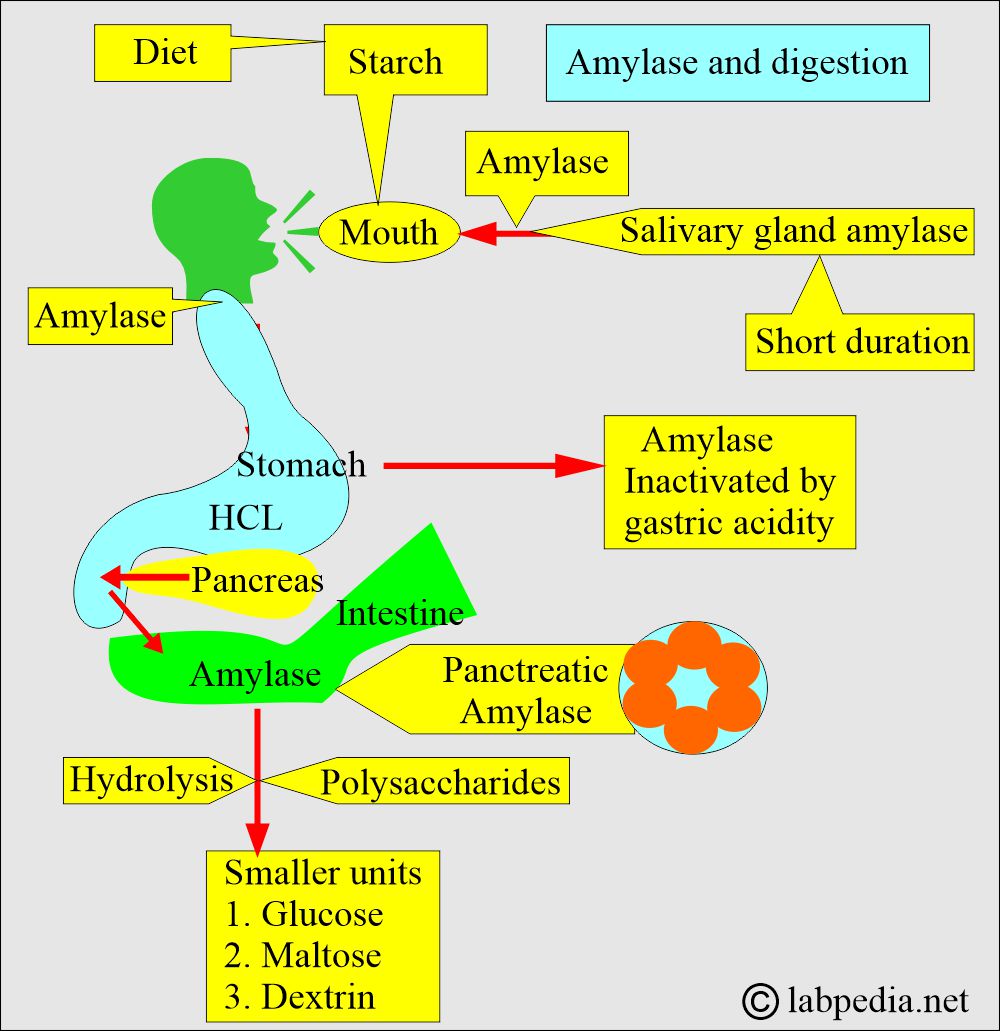
- In the duodenum, a slightly alkaline medium favors the effective action of pancreatic and intestinal amylase.
- The salivary glands also produce amylase to initiate the hydrolysis of starch while the food is still in the oral cavity and esophagus.
- Trypsin activity destroys amylase activity.
- Where will you find the amylase?
- Semen.
- Testes.
- Ovaries.
- Fallopian tubes.
- Milk.
- Colostrum.
- Tears.
- Striated muscles.
- Adipose tissue.
- Sometimes, the tumors of the lung and ovary may contain amylase activity.
- Ascitic and pleural fluid may contain amylase due to pancreatitis or neoplasm.
- The amylase in the serum and urine originates mainly from the pancreas and salivary glands.
- Serum amylase has an optimal pH range of 6.9 to 7.0. It is routinely tested at 37 °C but remains active at 50 °C.
Acute pancreatitis:
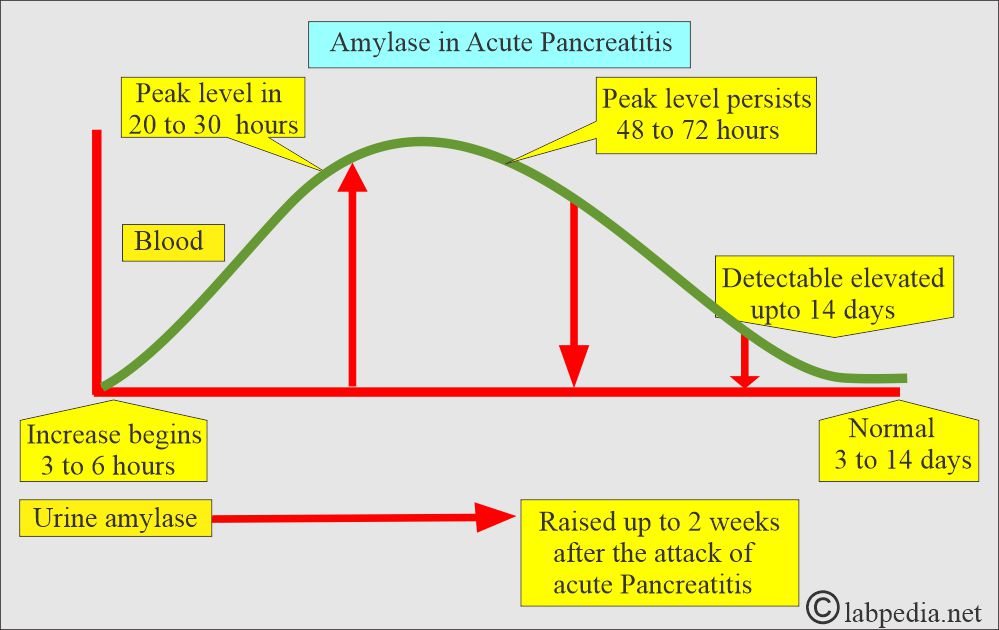
What are the changes in amylase in acute pancreatitis?
- The amylase enzyme is a good marker of acute pancreatitis.
-
- The initial rise is seen in 2 to 12 hours
- The peak level is 12 to 72 hours
- The normal level is seen after 3 to 14 days.
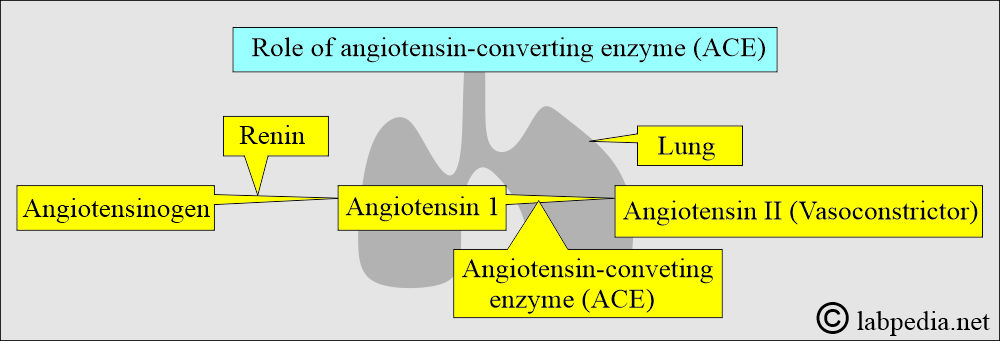
Angiotensin-converting enzyme (ACE)
How will you define Angiotensin-converting enzyme (ACE)?
- This enzyme is produced by endothelial and epithelial cells.
- This enzyme is also produced by activated macrophages in the granuloma.
What are the indications for angiotensin-converting enzyme?
- This test is used to monitor the disease’s activity.
- This enzyme is used to detect the clinical course of sarcoidosis.
- It differentiates between active and dormant sarcoidosis.
- It differentiates between sarcoidosis and other granulomatous diseases.
How will you describe the pathophysiology of ACE?
- This is found in the epithelial cells of the lungs.
- It converts angiotensin-1 to angiotensin II, which is a potent vasoconstrictor.
- It helps in controlling hypertension.
What are the causes of raised Angiotensin-converting enzyme (ACE)?
- Sarcoidosis. It is seen in 50% to 75% of cases of active pulmonary sarcoidosis.
- Other diseases showing a raised level of ACE are:
- Gaucher’s disease. It is raised in 100% of the cases.
- Leprosy.
- Tuberculosis.
- Alcoholic cirrhosis.
- Hodgkin’s lymphoma.
- Multiple myelomas.
- Scleroderma.
- Pulmonary embolism.
- Active histoplasmosis.
Questions and answers:
Question 1: What will be the level of acid phosphatase in children?
Question 2: How will you diagnose acute pancreatitis?