Diabetes Mellitus:- Part 2 – Diabetes Mellitus Diagnosis and Management
Diabetes Mellitus
What Sample for Glucose Estimation is needed?
- This test can be done on Serum. The Serum should be separated within 30 minutes of collection.
- The Serum can be stored at 25° C for 8 hours and 72 hours at 4 °C.
- Oxalated blood can also be used. Preservative sodium fluoride may be added.
- The plasma can be stored at 25 °C for 24 hours (with preservative sodium fluoride).
How much is the Stability of the sample for glucose?
- One ml of blood in anticoagulant will be stable for 3 hours with fluoride.
- Oxalate plasma is stable at 2 to 8 °C for 48 hours.
- Mostly Serum is used, stable for 8 hours at 25 °C and 72 hours at 4 °C.
- A fast of 6 to 8 hours is required for a fasting sample.
Indications for Diabetes Mellitus Patients:
- This test is done to diagnose diabetes mellitus.
- This test is also done to evaluate and monitor the patient with Diabetes mellitus.
What Screening indications for Diabetes are advised in individuals?
- People over the age of 45 years or older at 3-year intervals.
- Younger individuals should be screened if they are obese,>120% of the desired weight, or have a body index ≥ 27.
- Individuals with H/O first-degree relatives with Diabetes.
- In the case of high-risk ethnic groups, afro-American, Hispanic Americans, Native Americans, and Asian Americans.
- Babies delivered >9 Lbs of weight, and there is a previous H/O GDM.
- Individuals with hypertension ≥140/90 mm Hg and H/O atherogenic dyslipidemia.
- HDH-Cholesterol = ≤35 mg/dL.
- Triglycerides = ≥250 mg/dL.
How will you Define Diabetes mellitus?
- Diabetes mellitus is a group of metabolic disorders of carbohydrate metabolism in which glucose is not adequately utilized, leading to hyperglycemia.
- This is not a single disease but is a group of disorders with glucose intolerance in common.
- Diabetes mellitus describes a syndrome characterized by chronic hyperglycemia and disturbances of carbohydrates, protein, and fat metabolism.
- Diabetes Mellitus is a metabolic disorder characterized by hyperglycemia that results from defects in insulin secretion, insulin action, or both.
- This condition is also associated with protein and fat metabolism abnormality.
- Diagnosis is dependent upon hyperglycemia and glucosuria.
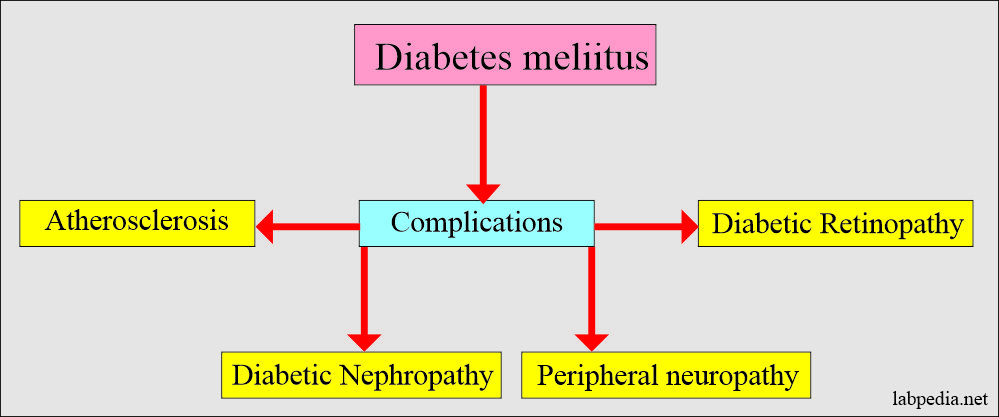
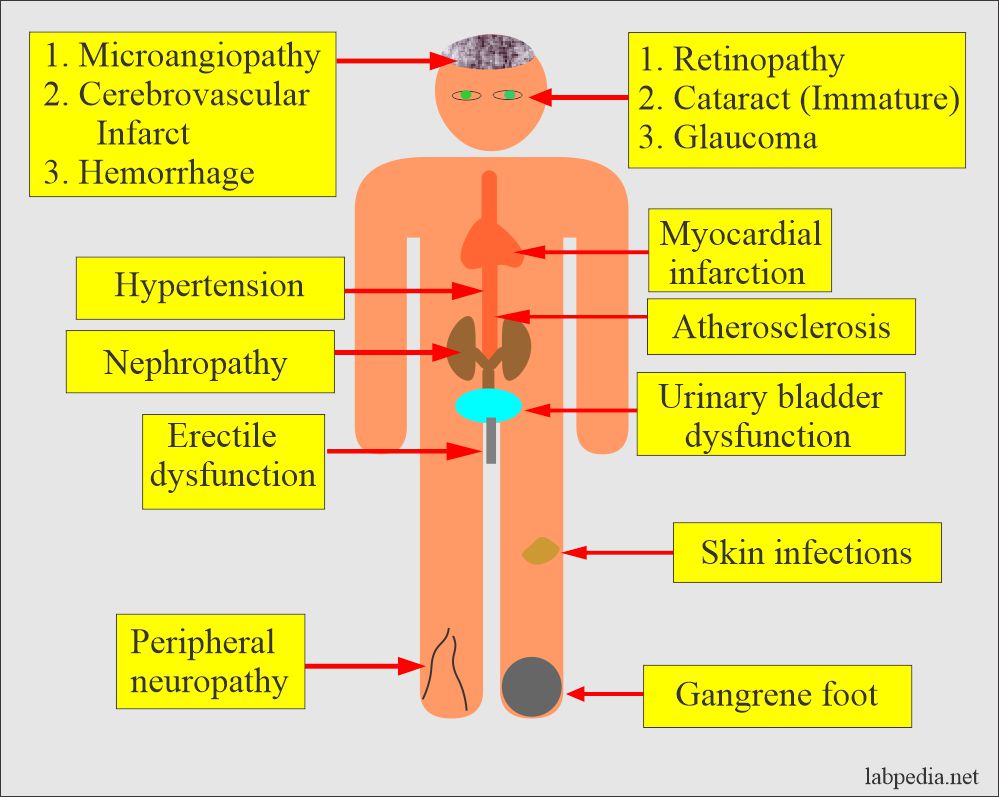
What are the Complications of diabetes mellitus?
- Chronic hyperglycemia leads to:
- Changes in the retina and lens of the eye (retinopathy)
- Damage to kidneys.
- Microalbuminuria.
- Nephropathy
- The heart, arterial system, and microcirculation are adversely affected.
- Increased risk of heart disease problems.
- These patients may develop neuropathy.
- The foot needs care and may develop gangrene.
- These patients may develop hearing problems.
- There are chances for Alzheimer’s disease.
Diabetes Mellitus
What are the Criteria for the Diagnosis of Diabetes Mellitus?
- Polyuria, polydipsia, and rapid weight loss.
- Fasting glucose level is high.
- Insulinopenia has decreased insulin due to the loss of β-cells in the pancreas.
- Most patients have autoantibody called an autoimmune process.
- When no cause is known is called idiopathic Type.
- Abnormal Glucose tolerance test.
Type of Diabetes Mellitus (classification of the diabetes mellitus):
- Type 1 diabetes mellitus (Insulin-dependent, IDDM).
- There is β-cell destruction, usually leading to absolute insulin deficiency.
- This may be immune-mediated.
- It may be Idiopathic.
- Type 2 diabetes mellitus (Noninsulin dependant, NIDDM).
- There is predominantly insulin resistance with relative insulin deficiency. OR
- There may be predominantly insulin secretion deficiency with insulin resistance.
- Gestational Diabetes Mellitus (Gestational diabetes mellitus, GDM).
- It is detected early in pregnancy. This may be type 1 or type 2.
- This is detected in the 2nd or 3rd trimester in 4% of pregnant ladies.
- Other specific types are:
- The genetic defects of β-cell dysfunction.
- The genetic defect in insulin action (Type A insulin resistance).
- Diseases of the pancreas (exocrine glands).
- Pancreatitis.
- Trauma or pancreatectomy.
- Tumor of the pancreas.
- Drugs or chemicals induced.
- Thiazide.
- Glucocorticoids.
- Nicotinic acid.
- Infections.
- Cytomegalovirus (CMV).
- Congenital rubella.
- Endocrinopathies.
- Glucagonoma.
- Cushing’s syndrome.
- Acromegaly.
- Immune-mediated diabetes.
- Genetic syndrome associated with diabetes mellitus are:
- Turner syndrome.
- Down’s syndrome.
- Myotonic dystrophy.
- Friedreich ataxia.
Diabetes Mellitus Type 1
Pathogenesis of type 1 diabetes mellitus:
- The autoimmune phenomenon may be the cause of type 1 diabetes mellitus:
- Type 1 diabetes mellitus is due to cell-mediated autoimmunity leading to the destruction of the insulin-secreting cells of the pancreatic β- cells.
- While other α, δ, and other islet cells are preserved.
- The islet cells have mononuclear cells infiltrated, called insulitis.
- The autoimmune process for type 1 diabetes begins years before the clinical presentation.
- An 80% to 90% reduction in the volume of β- cells is needed before clinical Diabetes appears.
- Destruction of the β- cells is more rapid in children than in adults.
- Antibodies that may play a role in type 1 diabetes are:
- There is a marker of β-cells autoimmunity where the antibodies in the Serum are detected before Diabetes appears.
- Islet cell cytoplasmic antibodies.
- Insulin auto-antibodies.
- Glutamic acid decarboxylase antibodies.
- There is a marker of β-cells autoimmunity where the antibodies in the Serum are detected before Diabetes appears.
- Genetic role:
- Type 1 diabetes is inherited, but the mode is not clear.
- Environmental factors:
- There are various factors reported, and one of those is the virus.
- Viruses like mumps, Bella, and coxsackievirus B are blamed.
- Other factors like cow’s milk and chemicals.
- This is because of the severe or absolute absence of insulin caused by the loss of beta cells in the pancreas.
- Destruction of the islet cells may be due to the following:
- Genetics.
- Autoimmunity.
- Environmental factors.
- In 80% to 90% of the cases, there are islet cell autoantibodies and antibodies to insulin and glutamic acid decarboxylase, which cause damage to the islet cells.
- Non-immune type 1 diabetes occurs secondary to other diseases like pancreatitis.
- Pathology: Beta-cell abnormalities are present long before the onset of type 1 diabetes mellitus.
- Both beta and alpha cell functions are abnormal, with a lack of insulin and a relative excess of glucagon produced by the alpha cells.
How will you define Type 1 Diabetes Mellitus?
- This is also called:
- Juvenile-onset Diabetes.
- Juvenile Diabetes.
- Ketosis prone diabetes.
- Brittle Diabetes.
- Autoimmune Diabetes.
- Idiopathic Diabetes.
- There is a long preclinical period with abrupt onset of clinical manifestations.
- Patients are prone to develop ketoacidosis.
- There is a dependency on insulin.
- This often affects young people around the age of puberty.
- The peak age of onset is 11 to 13 years.
- The risk for the sibling is 5 to 10%, while the risk for the offspring is 2 to 5%.
- There are several syndromes like autoimmune and genetic origin.
Signs and symptoms of diabetes mellitus Type 1:
- Glucose accumulates in the blood (hyperglycemia) and is excreted in the urine.
- There is weight loss due to the breakdown of proteins and fats.
- There is polyuria, polyphagia, and polydipsia.
- There is a wide fluctuation in the blood glucose level.
- There may be ketoacidosis because of the breakdown of protein and fat.
- There are increased ketone bodies.
- The pH drops, which triggers the buffer system and leads to metabolic acidosis.
-
- There is a fruity odor in the breath due to the volatile ketone body acetone.
-
- The patient may go into a coma.
Clinical manifestation and their explanation:
| Clinical manifestation | Explanation |
| Weight loss | There is a fluid loss due to osmotic diuresis and loss of body tissue as fat and protein are used for energy. |
| Fatigue | There are metabolic changes that result in poor food utilization, which will contribute to lethargy and fatigue. |
| Polyphagia | This is due to the depletion of the body’s fat, proteins, and carbohydrates leading to cellular starvation and increased hunger. |
| Polydipsia | This is due to a raised blood sugar level, which osmotically attracts the water from the cells, leading to intracellular dehydration and ultimately stimulating the hypothalamus and thirst. |
| Polyuria | Hyperglycemia acts as an osmotic diuretic and leads to Glycosuria, which is accompanied by water loss in the urine. |
What is the Treatment of Diabetes Mellitus Type 1?
- This will need a combination of the following:
- Insulin.
- Food planning.
- Exercise.
- More details are discussed at the end of this discussion.
Diabetes Mellitus Type 2 (NON-Insulin dependent NIDDM)
- This is also called:
- Adult-onset type diabetes.
- Maturity-onset Diabetes.
- Ketosis resistant diabetes.
- Patients have minimal symptoms.
- This is not dependent on insulin to prevent ketonuria.
- The insulin level may be normal, decreased or increased.
- Most patients have impaired insulin action.
- There is the interaction of metabolic, genetic, and environmental factors.
- It affects people after the age of 40 years, and mostly these are obese.
Pathophysiology of diabetes mellitus type 2:
- The cause is unknown.
- Genetics may play some role, but it is not clearly defined.
- There is no evidence of the autoimmune mechanism.
- Cellular resistance is a factor in 60% to 80% of people with type 11 diabetes mellitus.
- Insulin resistance increases with obesity.
- There is a decreased response of the β-cell to blood glucose levels and abnormal glucagon secretion.
- There may be alterations in the insulin-receptor or post-receptor events.
- There may be an increase in the insulin level to compensate for insulin resistance in the peripheral tissue, but still, there is relative insulin deficiency.
- The changes in the pancreas are nonspecific.
- 10% to 40% of the cases show amyloidosis of the pancreas in type 2 diabetes mellitus.
- Pancreatic fibrosis occurs in 33% to 66% of the cases with type 2 diabetes, leading to a decreased number of β-cells.
- Generally, there is a decrease in the weight and number of β-cells, and the cause is unclear.
- The most common factor is obesity. It increases 10 times in obese people.
- Also, excessive intake of calories predisposes to type 2 diabetes.
- Insulin can not facilitate the entry of glucose into the muscle cells, hepatocytes, and fat cells.
- One of the factors is the decreased ability of insulin to act on the peripheral tissue (insulin resistance).
Signs and symptoms of diabetes mellitus type 2:
- These are nonspecific.
- Most patients are obese and overweight.
- There is hyperlipidemia.
- Onset is slow and mostly not noted, which leads to late diagnosis.
- Classic symptoms like polydipsia, polyphagia, and polyuria are present.
- There may be nonspecific symptoms like pruritus, recurrent infections, paresthesia, and visual changes.
What is the Treatment of Diabetes Mellitus Type 2?
- This is just like type 1 diabetes. The aim is to keep blood sugar in the normal range.
- There is a need to decrease the calorie intake in an overweight person.
- Saturated fats and cholesterol are restricted.
- Some people recommend a high-fiber diet.
- Oral hypoglycemic drugs may be needed.
- Exercise also helps.
- Insulin may also be given.
Factors affecting glucose level:
- Stress like trauma, general anesthesia, infection, burns, and Myocardial infarction can Increase the glucose level.
- Caffeine may increase the level.
- Some pregnant women may experience glucose intolerance. A significantly raised level of glucose is called Gestational Diabetes.
- Drugs may increase the glucose level like an antidepressant (tricyclic), Beta-blockers, corticosteroids, I/V glucose, dextrothyroxine, diazoxide, diuretics, estrogen, glucagon, isoniazid, lithium, phenothiazine, phenytoin, and salicylates intoxication.
- Drugs like acetaminophen, alcohol, anabolic steroids, insulin, tolbutamide, propranolol, and clofibrate may decrease the glucose level.
What are the American diabetes association recommendations?
| Test | Normal | Goal |
| Glucose: Capillary whole blood, Preprandial | <100 mg/dL | 80 to 120 mg/dL |
| Average bedtime glucose | <120 mg/dL | 100 to 140 mg/dL |
| HbA1c | <6% | <7% |
What are Clinical manifestation and their explanation in Diabetes mellitus?
| Clinical manifestation | Explanations |
| Fatigue | This is due to the poor metabolism of the food products, which contributes to lethargy and fatigue. |
| Genital pruritus | Hyperglycemia and Glycosuria help the growth of fungal (candidiasis) infection, leading to pruritus, and most common in females. |
| Recurrent infection | There may be boil, carbuncle, and skin infections. The growth of the bacteria is enhanced by increased glucose. Also, the impaired blood supply helps the infection. |
| Prolonged wound healing | There is an impaired blood supply, which delays healing. |
| Paresthesia | This is due to diabetic neuropathy. |
| Eye changes | This is due to diabetic retinopathy. |
Gestational diabetes mellitus:
- Definition: Hyperglycemia develops for the first time during pregnancy.
- This is also called:
- Asymptomatic Diabetes.
- Chemical Diabetes.
- Borderline Diabetes.
- Latent Diabetes.
- Subclinical Diabetes.
- Gestational diabetes mellitus develops when glucose intolerance develops during pregnancy, so all pregnant women need to be tested.
- After the delivery, the glucose becomes normal, impaired, or progresses to Diabetes.
- This is first diagnosed during pregnancy and usually in the third trimester.
- Already known cases of diabetic women are not included in this group.
- This occurs in 6 to 8% of pregnant women (another source, only 2% of pregnant ladies may have this Diabetes).
- Out of this group, 60% may develop Diabetes in 15 years Of gestation.
- Later on, these ladies are at increased risk of developing diabetes mellitus (6 to 62% of these ladies).
- Risk factors in developing Gestational Diabetes are:
- Pregnant ladies with Glycosuria.
- If there is a family history of Diabetes.
- In obese ladies.
- If the ladies develop pregnancy at a late age.
- In multiparity of 5 or more.
- In the case of previous complicated pregnancies.
-
What are the diagnostic criteria for gestational diabetes mellitus:
- To diagnose gestational diabetes mellitus. Two blood samples on oral glucose tolerance tests (with 100 grams of glucose) are as follows:
| Blood sample timings | Blood glucose level |
| Fasting blood glucose | ≥95 mg/dL |
| One hour sample | ≥180 mg/dL |
| 2- hour sample | ≥155 mg/dL |
| 3-hour sample | ≥140 mg/dL |
- What is the Treatment of gestational diabetes mellitus?
- Advise random or fasting blood glucose during the pregnancy.
- It should be aggressive to prevent morbidity and fetal mortality.
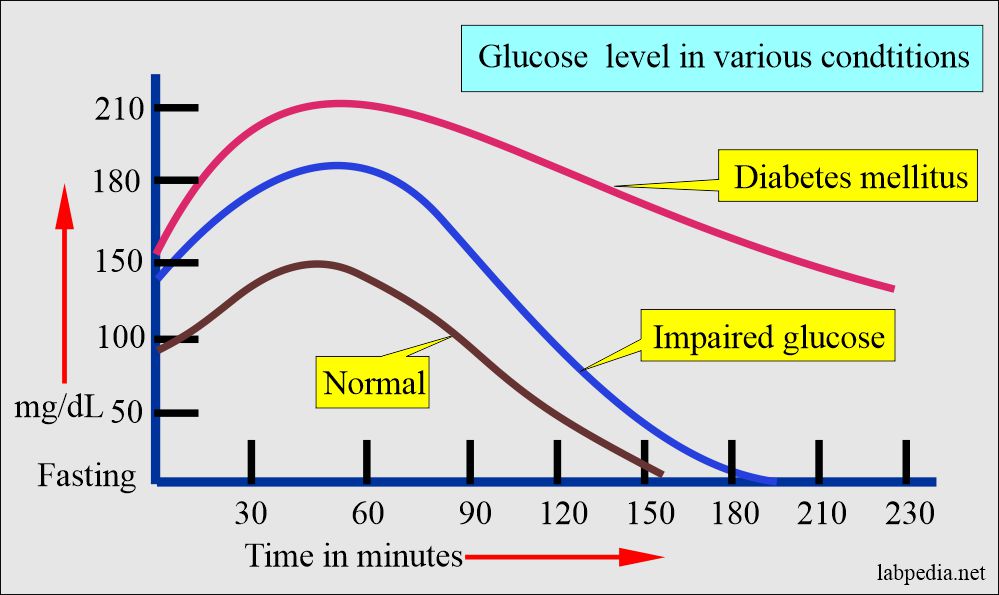
Impaired glucose tolerance (IGT)
- This group has less fasting glucose than required for diabetes mellitus.
- An oral glucose tolerance test is needed to diagnose this group.
- The overt case develops 1% to 5% per year.
- 10% to 20% will convert to type 11 diabetes within 10 years.
- Microvascular diseases are very uncommon in this group.
- Many of them are obese.
- What are the Criteria for impaired glucose tolerance?
- With an oral glucose tolerance test:
- 2-hour sample = ≥140 mg/dL and <200 mg/dL (nonpregnant ladies).
- With an oral glucose tolerance test:
Impaired fasting glucose (IFG)
- There is an abnormal response to an oral glucose tolerance test.
- What are the Criteria for the diagnosis of Impaired fasting glucose?
- Fasting glucose = ≥110 mg/dL and <126 mg/dL.
- 2 hours of glucose = ≥ 140 mg/dL.
- <200 mg/dL.
- This is diagnosed by fasting glucose values between normal and diabetic individuals.
- This is a metabolic stage between normal glucose and diabetes mellitus.
- There is an increased risk for the development of Diabetes and cardiovascular disease.
What are the Latest classification criteria for Diabetes mellitus?
- Diabetes mellitus:
- Presence of classic symptoms.
- If the fasting glucose level is 126 mg/dl (>7.0 mmol/L) or above, it should be labeled as D. Mellitus (when this value is found two times).
- One random glucose level of more > than 200 mg/dl (11.1 mmol/L) with symptoms of polyuria, polydipsia, and polyphagia is considered diagnostic of Diabetes.
- HbA1c is more than 6.5 % diagnostic for Diabetes.
- The 2-hour postprandial glucose level was≥200 mg/dl (11.1 mmol/L) during OGTT.
- Impaired fasting glucose = > 126 mg/dl. (fasting glucose level 110 to 125 mg/dL (6.1 to 7.0 mmol/L).
- Impaired glucose tolerance when:
- Fasting glucose < 126 mg/dl (7 mmol/L).
- OGTT 2-hour sample is 140 mg to 199 mg/dl (7.8 to 11.1 mmol/L).
What are the Criteria for the diagnosis of diabetes mellitus?
- Fasting blood glucose level:
- 126 mg/dL (7.0 mmol/L) or higher is considered diagnostic.
- Random/nonfasting blood glucose level:
- 200 mg/dL (11.1 mmol/L) is diagnostic.
- Oral glucose tolerance test with 75 G of glucose:
- A 2-hour sample of 200 mg/dL (11.1 mmol/L) or higher value is diagnostic.
What are the Values in diabetic patients and normal people?
| Diagnosis | Fasting glucose level | Random glucose level | 2-hour glucose level (in OGTT) | HbA1c |
| Normal | <100 mg/dL (5.6 mmol/L) | <14o mg/dL (7.8 mmol/L) | <5.7 | |
| Prediabetics | 100 to 125 mg/dL (5.6 to 6.9 mmol/L) | ≥140 to 199 mg/dL (7.8 to 11.0 mmoml/L) | ≥140 to 199 mg/dL (7.8 to 11.0 mmol/L) | 5.7 to 6.4% |
| Diabetes mellitus | ≥ 126 mg/dL (7.0 mmol/L) | 200 mg/dL (11.1 mmom/L) | ≥200 mg/dL (11,1 mmol/L) | ≥6.5% |
What are the Differences between Diabetes Mellitus type 1 and type 2?
| Parameters | Type 1 diabetes mellitus | Type 2 diabetes mellitus |
| Presentation |
|
|
| Insulin level |
|
|
| Genetic role | 40% seen in the twins | 60 to 80% seen in the twins |
| Pathogenesis |
|
|
| Biochemical difference | Ketoacidosis is common | Ketoacidosis is rare |
What is the normal fasting glucose level?
Source 1
| Age | mg/dL |
| Cord blood | 45 to 96 |
| Premature | 20 to 60 |
| Neonates | 30 to 60 |
| Newborn 1 day | 40 to 60 |
| >one day | 50 to 80 |
| Child | 60 to 100 |
| Adult | 74 to 104 |
| 60 to 90 years | 82 to 115 |
| >90 years | 75 to 121 |
- To convert to SI units x 0.0555 = mmol/L
- Values vary from the biochemical method used.
Source 6 for glucose level
| Blood glucose fasting | mg/dL | mmol/L |
| Cord | 45 to 96 | 2.5 to 5.3 |
| Premature infants | 20 to 60 | 1.1 to 3.3 |
| Neonatal | 30 to 60 | 1.7 to 3.3 |
| Infants | 40 to 90 | 2.2 to 5.0 |
| Child <2 years | 60 to 100 | 3.3 to 5.5 |
| Child >2 years to adult | ||
| Fasting | 70 to 100 | <6.1 |
| Elderly | Increase after 50 years |
Source Tietz
Plasma/ serum glucose level
- Adult = 74 to 106 mg/dL (4.5 to 5.9 mmol/L)
- Children = 60 to 100 mg/dL (3.5 to 5.6 mmol/L)
- Premature neonates = 20 to 60 mg/dL (1.1 to 3.3 mmol/L)
- Term neonates = 30 to 60 mg/dL (1.7 to 3.3 mmol/L)
The whole blood glucose level
- 65 to 95 mg/dL (3.5 to 5.3 mmol/L)
CSF glucose level
- 40 to 70 mg/dL (2.2 to 3.9 mmol/L)
- 60% of the plasma
Urine 24 hours glucose level
1 to 15 mg/dL (0.1 to 0.8 mmol/L)
The normal value of glucose from another source:
- Usually, glucose between 70 to 110 mg/dl is considered normal.
- Fasting glucose = < 100 mg/dl.
- Cord blood = 45 to 96 mg/dL (2.5 to 5.3 mmol/L)
- premature baby = 20 to 60 mg/dL. (1.1 to 3.3 mmol/L).
- Neonates = 30 to 60 mg/dL (1.7 to 3.3 mmol/L).
- Infants = 40 to 90 mg/dL (2.2 to 5.0 mmol/L).
- Child <2 years = 60 to 100 mg/dL (3.3 to 5.5 mmol/L).
- Child >2 years = like adult level.
- Adult fasting = 70 to 110 mg/dL (<6.1 mmol/L).
- Adult random = <160 mg/dL (11.1 mmol/L).
Various types of diabetes mellitus and glucose values:
| Diagnosis | Fasting glucose level | Random/non-fasting glucose level | 2 hours glucose after 75 grams of oral test |
| Diabetes mellitus | >125 mg/dL | >199 mg/dL (classic S/S and glucose ≥200 mg/dL) | >199 mg/dL |
| Pre-diabetes (impaired fasting glucose) | >99 mg and <125 mg/dL | – | ≥140 to <200 mg/dL |
| Pre-diabetes (impaired glucose tolerance) | <126 mg/dL | >139 mg and <200 mg/dL | |
| Gestational diabetes | >105 mg/dL |
|
Glucose values in whole blood child/adult:
| Fasting | Child mg/dL | Adult mg/dL |
| Serum or plasma | 60 to 105 | 70 to 100 |
| Whole blood | 50 to 90 | 60 to 100 |
| 2 hours, postprandial | ||
| Serum or plasma | around 150 | around 140 |
| Whole blood | around 120 | around 120 |
Diabetes Mellitus classification based on oral 75 G Glucose overload:
| Patterns of Glucose | Fasting glucose mg/dL | Postprandial glucose mg/dL | 2 hours of glucose mg/dL |
| Normal | <115 | <200 | <140 |
| Diabetes Mellitus | >140 | >200 | >200 |
| Impaired glucose tolerance | <140 | >200 | 140 to 190 |
Critical values of Glucose:
| Age | Critical low glucose level mg/dL | Critical high glucose level mg/ dL |
| Adult male | < 50 | > 400 |
| Adult female | < 40 | > 400 |
| Infants | < 40 | |
| Newborn | < 30 | > 300 |
What are the causes of raised glucose levels (Hyperglycemia)?
- Diabetes mellitus, adult, and juvenile.
- Physiological causes.
- Strenuous exercise.
- Strong emotions.
- Shock and burns.
- Infections.
- Endocrine disorders.
- Thyrotoxicosis
- Acromegaly and gigantism.
- Pheochromocytoma.
- Cushing’s syndrome.
- Pancreatic diseases.
- Acute and chronic pancreatitis.
- Pancreatitis due to mumps.
- Cystic fibrosis.
- Hemochromatosis.
- Pancreatic cancers.
- Other causes are:
- Cerebrovascular accident.
- Chronic liver disease.
- Chronic renal disease.
- Acanthosis nigricans.
What are the causes of decreased glucose levels (Hypoglycemia)?
- Pancreatic disorders.
- Islet Cell Tumor.
- Glucagon deficiency.
- Tumors.
- Adrenal gland carcinoma.
- Carcinoma of the stomach.
- Fibrosarcoma.
- Liver diseases.
- In poisoning, e.g., arsenic, chloroform, carbon tetrachloride, phosphorus, salicylates, antihistamines, phenformin, and alcohol.
- Endocrine disorders.
- Hypopituitarism.
- Addison’s disease.
- Hypothyroidism.
- Functional disorders.
- Postgastrectomy.
- Gastroenterostomy.
- Autonomic nervous system disorders.
- Pediatric causes.
- Prematurity.
- Infant diabetic mothers.
- Idiopathic leucine sensitivity.
- Enzyme deficiency.
- Galactosemia.
- Fructose intolerance.
- Von Gierke’s syndrome.
What are the complications of Diabetes Mellitus?
Acute complications are:
- There may be hypoglycemia.
- Patients with uncontrolled hyperglycemia of Type I may develop life-threatening complications like diabetic Ketoacidosis.
- Without treatment, the patient may become acidotic and dehydrated and lose consciousness.
- Type II may develop hyperosmolar coma.
Chronic complications are:
- Peripheral neuropathy.
- Diabetic retinopathy and cataract formation.
- Cardiovascular microangiopathy.
- Coronary atherosclerosis.
- Myocardial infarction is 3 to 5 times more common in diabetic patients.
- AMI is the leading cause of death in diabetes mellitus type 2.
- Peripheral vascular diseases like ischemia of lower extremities, erectile dysfunction, and intestinal ischemia.
- Gangrene of the foot.
- Diabetic kidney disease (diabetic nephropathy) may lead to end-stage renal disease.
- Chronic pyogenic skin infection.
- Candidal infection of the skin.
- Bone and joints show contracture.
How will you monitor the Diabetes mellitus patients?
- In the newly diagnosed patient, check glucose frequently.
- The best times are:
- Before meals.
- At bedtime.
- The goal of therapy is:
- To maintain euglycemia.
- Avoid hypoglycemia.
- Prevent cardiovascular diseases.
- Prevent neurological complications.
How will you treat the Diabetes mellitus patients?
- It requires a number of modalities to treat diabetic patients:
- Diet control.
- This includes dietary fibers in the diet.
- Eat low glycemic index foods, which will not raise blood glucose. This glycemic index is 55 or low, including vegetables, fruits, pasta, grainy bread, and legumes.
- High glycemic index foods have a value above 77 or greater. This will include potatoes, white bread, and white rice.
- The addition of protein and fats can lower the Glycemic index.
- Artificial sweeteners can be used in cooking and baking.
- Fructose is a natural sweetener and does not increase glucose levels.
- Medications to lower hyperglycemia are:
- The first-generation sulphonylureas are tolbutamide, tolazamide, acetohexamide, and chlorpropamide.
- Second-generation sulphonylureas are glyburide, glipizide, gliclazide, and glimepiride.
- Repaglinide.
- Nateglinide.
- Drugs that lower the glucose level by their action on the liver, muscle, and adipose tissue are:
- Metformin.
- Thiazolidinediones.
- Medications that affect the absorption of glucose are:
- Acarbose.
- Miglitol.
- Incretins are oral insulin stimulators:
- GLP-1 receptor antagonists.
- DPP-4 inhibitors.
- Sodium-glucose co-transporter 2 inhibitors.
- Insulin has various preparations.
- Transplant of the pancreatic tissue.
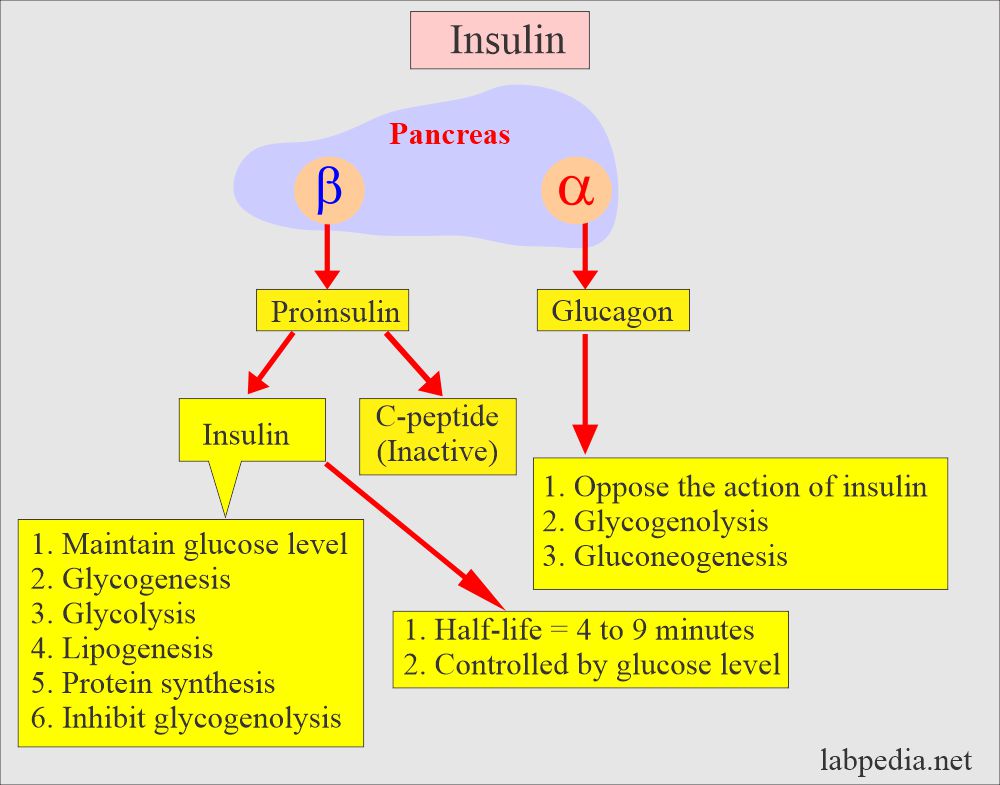
Functions of various Hormones related to glucose produced by the pancreas:
| Chemical substance | Clinical significance |
| Insulin |
1. Evaluation of fasting hypoglycemia 2. Evaluation of polycystic ovary 3. Classification of Diabetes mellitus 4. Predict diabetes mellitus 5. Assessment of β-cell activity 6. To find the insulin resistance |
| Proinsulin |
1. Diagnose the β-cell tumors 2. Cross-reactivity of insulin in different methods 3. Diagnosis of familial hyperinsulinemia |
| C-peptide |
1. Evaluation of Fasting hypoglycemia 2. Evaluation of β-cell tumors and beta-cell activity 3. Classification of Diabetes mellitus 4. Monitoring the patient with pancreatectomy and transplant of pancreas islet cells |
| Glucagon | For the diagnosis of α- cell tumors |