Chloride (Blood Chloride Cl¯ ) and Cystic Fibrosis
Blood Chloride (Cl–)
Sample for Blood Chloride Cl¯
- It is done on the serum or plasma of the patient.
- Except for emergency collect fasting samples because there is a slight decrease after the meal.
- Chloride is estimated in sweat to rule out cystic fibrosis.
- Other samples are CSF and 24 hours of urine.
Purpose of the test (Indications)
- As a part of electrolytes, acid-base balance.
- It gives status for hydration.
- For the diagnosis of cystic fibrosis.
Precautions for Blood Chloride (Cl–)
- Separate serum or plasma from the cells, as a change in pH, will alter the distribution of Chloride.
- Avoid hemolysis.
- Serum, plasma, and urine are stable for one week at 1 to 4 °C or room temperature.
- A frozen sample can be kept for one year.
- Drugs that may increase the chloride level are ammonium chloride, acetazolamide, cortisones, androgens, and estrogens.
- Drugs that may decrease the chloride level are aldosterone, corticosteroids, thiazide diuretics, and loop diuretics.
Definition of blood chloride (Cl–):
- Chloride is the most abundant extracellular anion.
- The same conditions affect chloride, which affects sodium (the most abundant extracellular cation).
Pathophysiology of Blood Chloride (Cl–)
- Chloride is the most abundant extracellular anion.
- Chloride is the major negative electrolyte(anion) in the extracellular fluid.
- Chloride with sodium represents the majority of the osmotically active constituents of plasma. Thus, the serum chloride level changes in the same direction as the sodium level, except for a few conditions. If the serum sodium is low, then serum chloride will also be low.
- The interstitial plasma fluid of chloride anion is 103 mmol/L.
- Its intracellular fluid (RBC) concentration is 45 to 54 mmol/L.
- While the intracellular fluid of other tissue is only 1 mmol/L.
- Most physicians advise “electrolytes panel or profile,” which includes:
- Sodium.
- Potassium.
- Chloride.
- Bicarbonate.
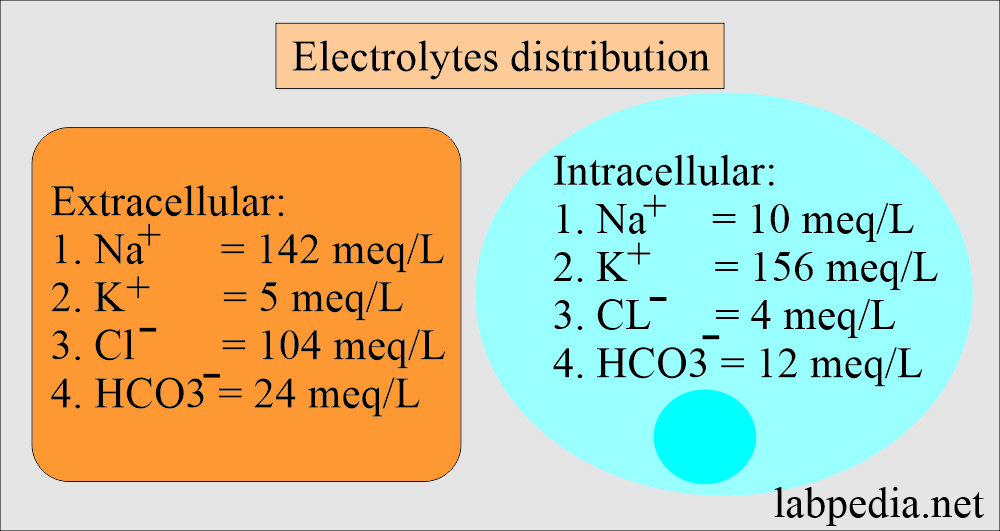
| Electrolytes | Extracellular fluid (ECF) meq/L | Intracellular fluid (ICF) meq/L |
| Sodium | 142 | 10 |
| Potassium | 5 | 156 |
| Chloride | 104 | 4 |
| Bicarbonate | 24 | 12 |
Chloride absorption and excretion:
- Chloride ions in the food are absorbed entirely in the intestine.
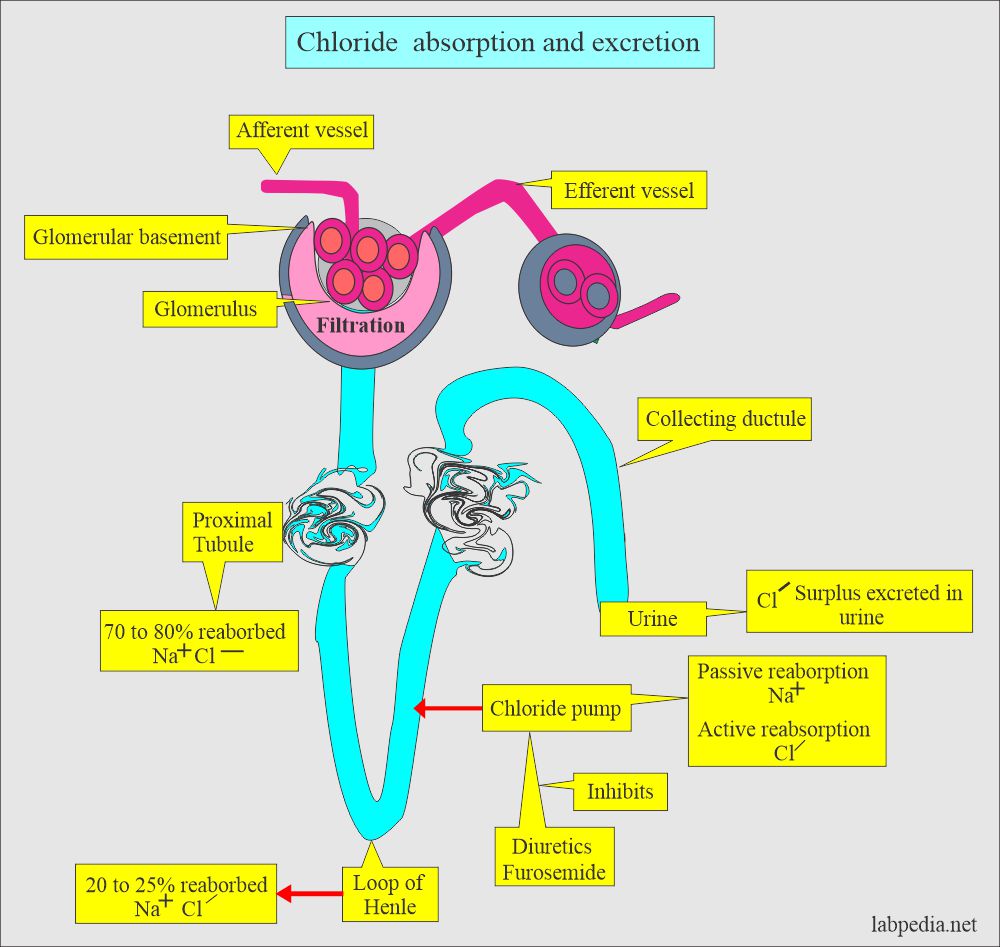
- They are filtered from the plasma at the glomerular level and passively reabsorbed, along with Na+ in the proximal tubules.
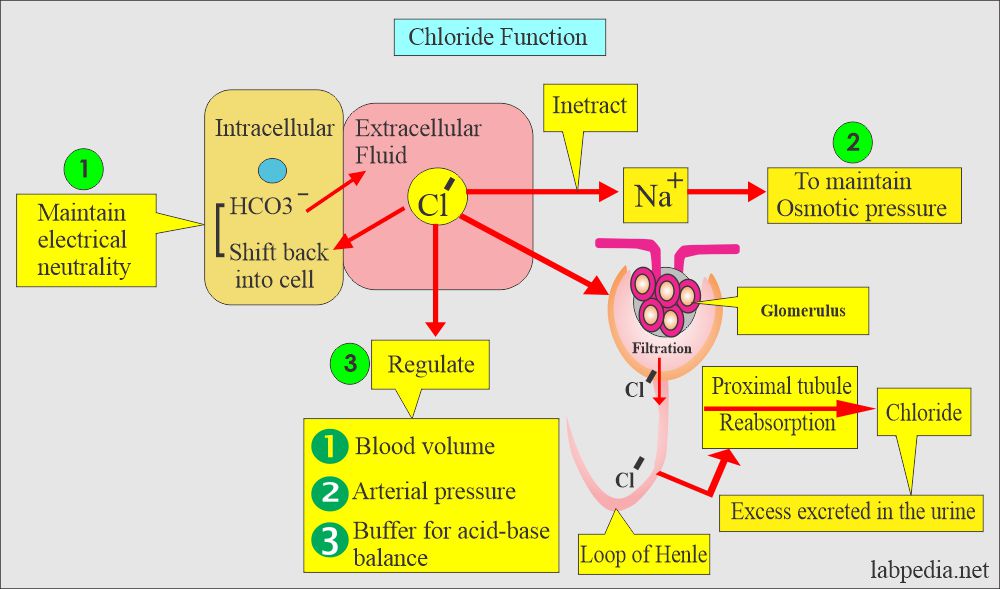
- Chloride interacts with sodium to maintain the osmotic pressure of blood.
- Its main purpose is to maintain the electrical neutrality of salt with sodium.
- Aldosterone increases the reabsorption of sodium and Chloride to maintain neutrality.
- Chloride acts as a buffer to help in acid-base balance.
- The concentration of the Cl– changes inversely with the changes in the concentration of HCO3–.
- Chloride is filtered at the glomerulus passively and reabsorbed at proximal tubules. Further absorption at the loop of Henle.
- There is a chloride pump in the ascending limb of the loop of Henle.
- Sodium is absorbed passively, while Chloride is absorbed actively by the pump.
- Excess Chloride is excreted in the urine and sweat.
- The chloride concentration in the urine is important in diagnosing metabolic alkalosis.
- Metabolic alkalosis can be corrected with saline I/V therapy in case of decreased extracellular water and where the urine concentration of chloride is <15 mmol/L.
- Metabolic alkalosis with normal extracellular water will have urine chloride >15 mmol/L and will not respond to saline therapy.
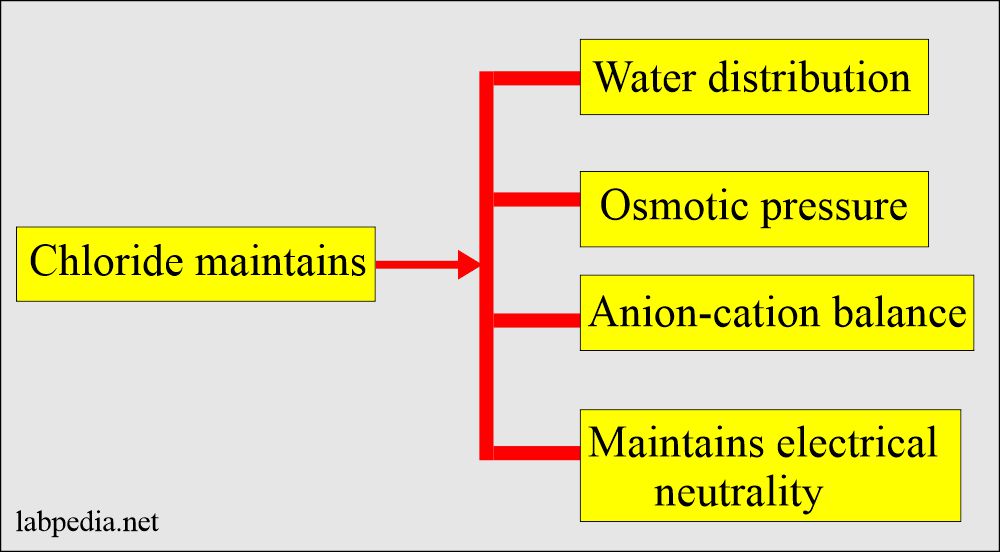
Functions of Blood Chloride (Cl–):
- Maintenance of water balance and osmotic pressure with the help of sodium.
- Chloride moves into cells in exchange for bicarbonate produced in the cells.
- It can maintain electrical neutrality.
- It helps as a buffer to help in the acid-base balance.
- Anion-cation balance in the extracellular fluid compartment.
- Chloride provides electroneutrality, particularly with Na+.
The normal level of Blood Chloride (Cl–)
Source 1
- Serum = 95 to 105 meq / L (98 to 106 mmol/L)
- Urine = 110 to 250 meq/ 24 hours
- Sweat:
- Normal = 5 to 40 meq/L
- Marginal value = 30 to 70 meq/L
- Cystic fibrosis = 60 to 200 meq/L
- CSF :
- Infant = 110 to 130 meq/L
- Adult = 118 to 132 meq/L
- These are 15% higher than those in serum.
- Saliva without stimulation = 5 to 20 meq/L
Other Sources
| Sample | meq/L |
| Serum or plasma | |
| Cord blood | 96 to 104 |
| Premature infant | 95 to 110 |
| 0 to 30 days | 98 to 113 |
| Adult | 98 to 107 |
| >90 years | 98 to 111 |
| Urine 24 hours | meq/24 hours |
| Infants | 2 to 10 |
| <6 years | 15 to 40 |
| Male 6 to 10 years | 36 to 110 |
| Female 6 to 10 years | 18 to 74 |
| Male 10 to 14 years | 64 to 176 |
| Female 10 to 14 years | 36 to 173 |
| Adult | 110 to 250 |
| >60 years | 95 to 195 |
| Cerebrospinal fluid | meq/L |
| Infant | 110 to 130 |
| Adult | 118 to 132 |
| Feces 24 hours | meq/L |
| 3.2 to ± 0.7 | |
| Sweat | meq/L |
| Normal | 5 to 35 |
| Marginal | 30 to 70 |
| Cystic fibrosis | 60 to 200 |
| Saliva | meq/L |
| normal without stimulation | 5 to 20 |
Cystic fibrosis
- Cystic fibrosis (mucoviscidosis) or fibrocystic disease of the pancreas is the most common lethal autosomal recessive. an inherited disorder in Europe.
- In Europe, it is estimated 1 in 2,000 live births.
- In Afro-Americans, the incidence is 2% of that in Europe.
- It is rare in Asians.
Pathology of cystic fibrosis:
- Around 90% of the homozygotes have symptoms predominantly resulting from damage to mucus-producing glands.
- Non-mucus-producing glands may also be affected.
- The mucus is very thick, which can plug the ducts and lead to obstructive complications.
- In the lungs, it leads to recurrent bronchopneumonia. This is a very serious complication.
- Pseudomonas and staphylococcus aureus are the common pathogens.
- The next complication is the complete or partial destruction of the exocrine portion of the pancreas, and this will end up in various conditions like:
- Malabsorption.
- Steatorrhea.
- Digestive disturbances.
- Malnutrition.
- A less common complication is biliary cirrhosis.
Sweat test for cystic fibrosis:
Procedure to collect the sweat:
- Sweat is collected in a plastic bag or by iontophoresis.
- Sweat is induced by sweat-stimulating agents like the pilocarpine.
- Iontophoresis consists of two small electrodes that create a tiny electric current.
- Sweat is collected in the small gauze pad.
- This procedure is painless.
Sweat test interpretation:
- The level of sodium and chloride is higher in patients with cystic fibrosis.
- For a definite diagnosis, sweat is collected. The sample should weigh >50 mg, and weighing <50 mg is inadequate.
- In children, sweat sodium >70 meq/L and chloride >60 meq/L are abnormal and are diagnostic of cystic fibrosis.
- Sodium and chloride may be raised in the first three days of life and then decrease to childhood level by 4th day.
- The sample should not be collected from the palm as there is an increased concentration of sodium and chloride (electrolytes).
Signs and symptoms of Hypochloremia:
- There is the loss of Cl–, usually resulting from hyponatremia or elevated HCO3– concentration, as in metabolic alkalosis.
- This will develop with vomiting and loss of HCL.
- Hypochloremia characterizes cystic fibrosis.
- Restricted use of salt or in use of diuretics is accompanied by Cl– deficiency.
- There is hyperstimulation of the nervous system and muscles.
- Shallow breathing.
- Hypotension.
- Tetany.
Signs and symptoms of Hyperchloremia
- This occurs when there is too much sodium or too little bicarbonate.
- More than a normal amount of Cl – can be expected with hypernatremia or metabolic acidosis.
- Ingestion of excessive Cl– accompanies the use of an ammonium chloride diuretic.
- Usually, no specific symptoms are associated with chloride excess.
- There are lethargy and weakness.
- Deep breathing.
Increased level of Blood Chloride (Cl–) (Hyperchloremia):
- urinary tract obstruction, glomerulonephritis, renal tubular acidosis, and acute renal failure.
- Diabetes Insipidus.
- Salicylate intoxication.
- Prolonged diarrhea with the loss of sodium bicarbonate.
- Respiratory alkalosis.
- Some cases of primary hyperparathyroidism.
- Maybe because of excessive intake.
- Eclampsia.
- Cushing syndrome.
- Renal tubular acidosis.
- Dehydration.
- Due to the excessive infusion of normal saline.
- Hyperventilation.
Decreased level of Blood Chloride (Cl–) (Hypochloremia):
- excessive sweating.
- Prolonged vomiting.
- Gastric suction.
- Salt losing nephritis.
- Addisonian crises.
- Metabolic acidosis is associated with increased organic anions.
- Aldosteronism.
- Respiratory acidosis.
- Water intoxication.
- Diuretic therapy.
- Hypokalemia.
- Burn
- Overhydration.
Serum electrolytes in various conditions:
| Clinical condition | pH | Chloride meq/L | Sodium meq/L | Potassium meq/L | Bicarbonate meq/L |
| Normal | 7.35 to 7.45 | 100 to 106 | 136 to 145 | 3.5 to 5.0 | 24 to 26 |
| Diabetic acidosis | 7.2 | 80 | 122 | 5.6 | 10 |
| Severe diarrhea | 7.2 | 96 | 128 | 3.2 | 12 |
| Vomiting | 7.6 | 94 | 150 | 3.2 | 38 |
| Respiratory acidosis | 7.1 | 80 | 142 | 5.5 | 30 |
| Respiratory alkalosis | 7.6 | 112 | 136 | 5.5 | 14 |
Acidosis and alkalosis:
| Clinical conditions | pH | Chloride meq/L | Bicarbonate meq/L | Sodium meq/L | pCO2 (mm Hg) |
| Normal | 7.40 | 105 | 25 | 14040 | |
| Metabolic acidosis | 7.30 | 115 | 15 | 140 | 31 |
| Metabolic alkalosis | 7.49 | 92 | 36 | 140 | 48 |
| Respiratory alkalosis (Chronic) | 7.44 | 102 | 25 | 136 | 40 |
| Respiratory acidosis (Chronic) | 7.37 | 100- 102 | 28 | 140 | 50 |
|
7.39 | 108 | 14 | 136 | 24 |
|
7.4 | 90 | 40 | 140 | 67 |
|
7.4 | 103 | 25 | 140 | 40 |
Critical values of chloride in serum or plasma are:
- High value = >115 meq/L.
- Low value = <80 meq/L.
Questions and answers:
Question 1: What is iontophoresis?
Question 2: How much is the chloride in the cells (intracellular)?