Calcium: – Part 1 – Calcium Total, Hypercalcemia and Hypocalcemia
Calcium Total
Sample for Calcium Total
- It is done on the serum of the patient.
- The blood should be collected without much pressure on the arm.
- Avoid prolonged tourniquet.
- EDTA cannot be used as an anticoagulant for the plasma.
- Obtain blood with minimal venous occlusion and without exercise or after restoring circulation.
- The serum is stable for 8 hours at 22 to 25 °C. But can keep at 4 °C for a longer period.
Purpose (Indications) of Calcium Total
- The serum level of calcium is used to evaluate parathyroid function and metabolism.
- Serum calcium level is used to monitor renal failure and renal transplantation.
- Serum calcium level is used to evaluate hyperparathyroidism.
- Serum calcium levels may be done in malignancies like multiple myeloma.
- Serum calcium levels may be done to monitor calcium levels before and after blood transfusions.
- It is advised following thyroidectomy and parathyroidectomy.
- It is advised in acute pancreatitis.
- It is advised in various drugs to see their effect.
Precautions for Calcium Total
- Venous stasis during blood collection by prolonged tourniquet application increases the calcium level.
- A fasting specimen is preferred.
- Venous stasis or erect posture increased the calcium level by 0.6 mg/dL.
- Diurnal variation is higher in PM (around 9 PM) than in AM (lowest).
- Separate serum immediately from RBCs to avoid calcium uptake by these cells (RBCs).
- Excessive intake of milk leads to increased calcium levels.
- Vitamin D intoxication also increases the calcium level.
- Check the albumin level because hypoalbuminemia leads to an artificial decrease in the calcium level.
- Drugs like calcium salts, alkaline antacids, thiazide diuretics, vitamin D, parathyroid and thyroid hormones, and androgens may increase the serum calcium level.
- Drugs like aspirin, anticonvulsants, heparin, laxatives, diuretics, magnesium salts, and oral contraceptives may decrease the calcium level.
- Calcium is increased by hyperalbuminemia, like in multiple myeloma and Waldenstrom macroglobulinemia.
- It is increased by dehydration.
- Hyponatremia (<120 meq/L) increases the protein-bound fraction of calcium. At the same time, hypernatremia decreases serum calcium.
- The hemodilution decreases serum calcium.
Definition of Calcium (Total):
- Calcium (Ca ++) is our body’s 5th most common element and most common cation in our body.
- The average human body contains around 1 kg (24.95 mol) of calcium.
- Calcium is found in the skeleton, soft tissue, and extracellular fluid.
- A calcium daily intake of calcium is about 400 mg is needed by the body.
- The minerals required by our body are:
- Sodium.
- Potassium.
- Calcium.
- Chloride.
- Phosphorus.
- Magnesium.
- Organically bound-S.
- Other elements required in trace amounts are:
- Iron.
- Zinc.
- Copper.
- Manganese.
- Selenium.
- Chromium.
- Molybdenum.
- Cobalt.
- Iodine.
Calcium Metabolism:
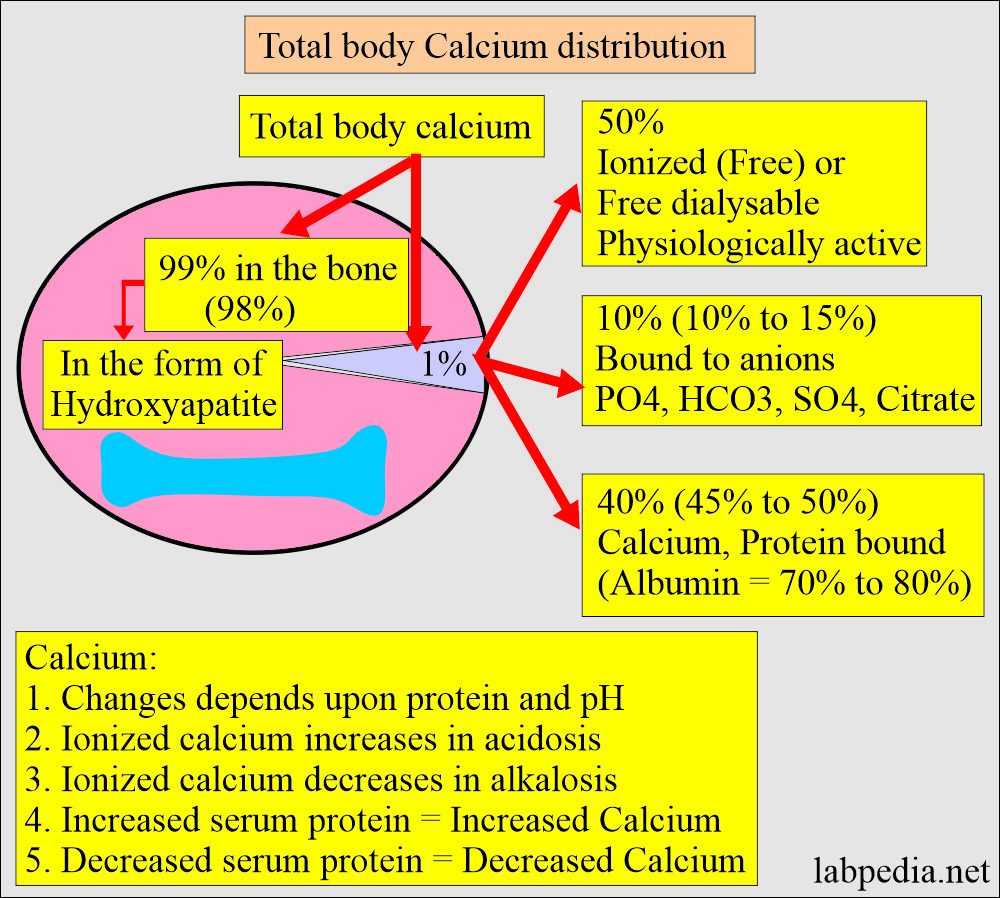
Calcium distribution:
- There is a large amount of calcium in the body, mainly in the bones and teeth.
- About 99% of calcium is deposited in the skeleton as a mixture of:
- Amorphous calcium phosphate.
- Crystalline hydroxyapatite.
- Calcium phosphate crystal (hydrated).
- A small amount of fluoride is incorporated into the calcium phosphate in the teeth and bone.
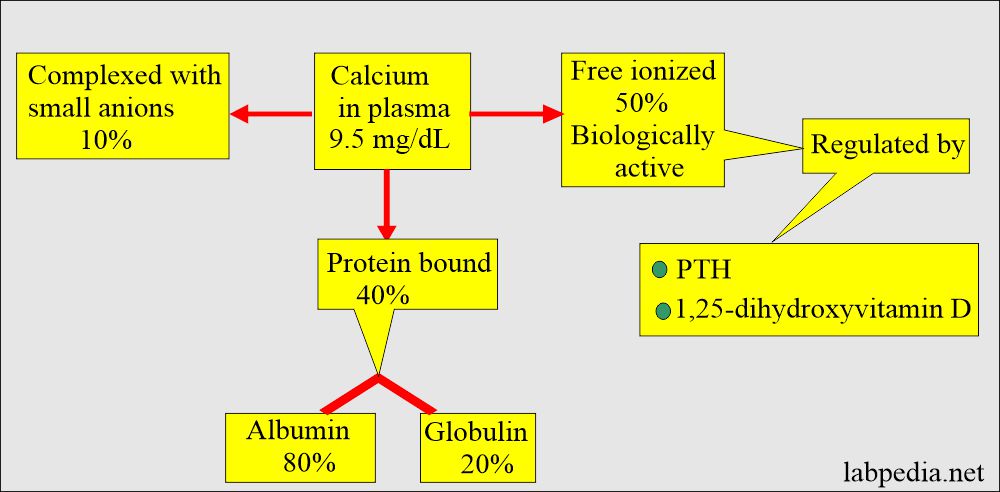
- Half of the calcium in blood circulation is free in ionized form, and half is protein-bound, mostly with albumin.
- 50% free or ionized (active) form of calcium.
- 40% is bound to the protein of calcium.
- 80% of calcium is bound to albumin, and 20% is bound to globulin.
- 10% is complex with anions.
- Some physician prefers ionized serum calcium level to avoid the effect of albumin level.
- Complexed calcium is complexed with small diffusible anions:
- Bicarbonate.
- Lactate.
- Phosphate.
- Citrate.
- Calcium in the blood is virtually all present in the plasma.
- It increases in acidosis and decreases in alkalosis.
- An increase in the plasma proteins leads to an increase in serum total calcium.
- Decreased plasma proteins lead to a decrease in the total serum calcium.
- Half of the calcium in blood circulation is free in ionized form, and half is protein-bound, mostly with albumin.
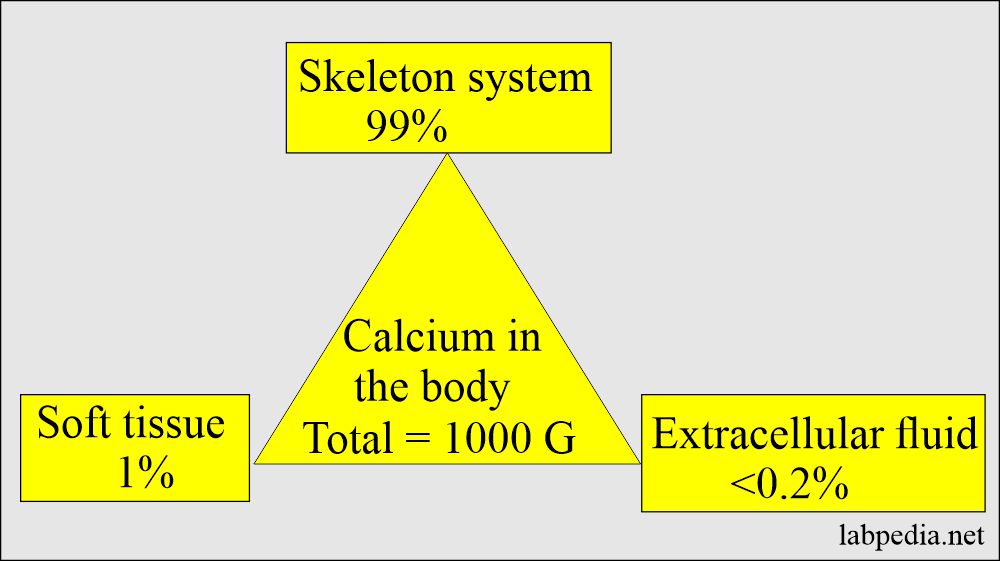
Distribution of calcium in the body:
| Presence of Calcium in the body | Calcium amount | Phosphate amount |
| Total calcium | 1000 g | 600 g |
| Extracellular fluid | <0.2% | <0.1% |
| Soft tissue | 1.0% | 15.0% |
| Skeleton (bones) | 99.0% | 85.0% |
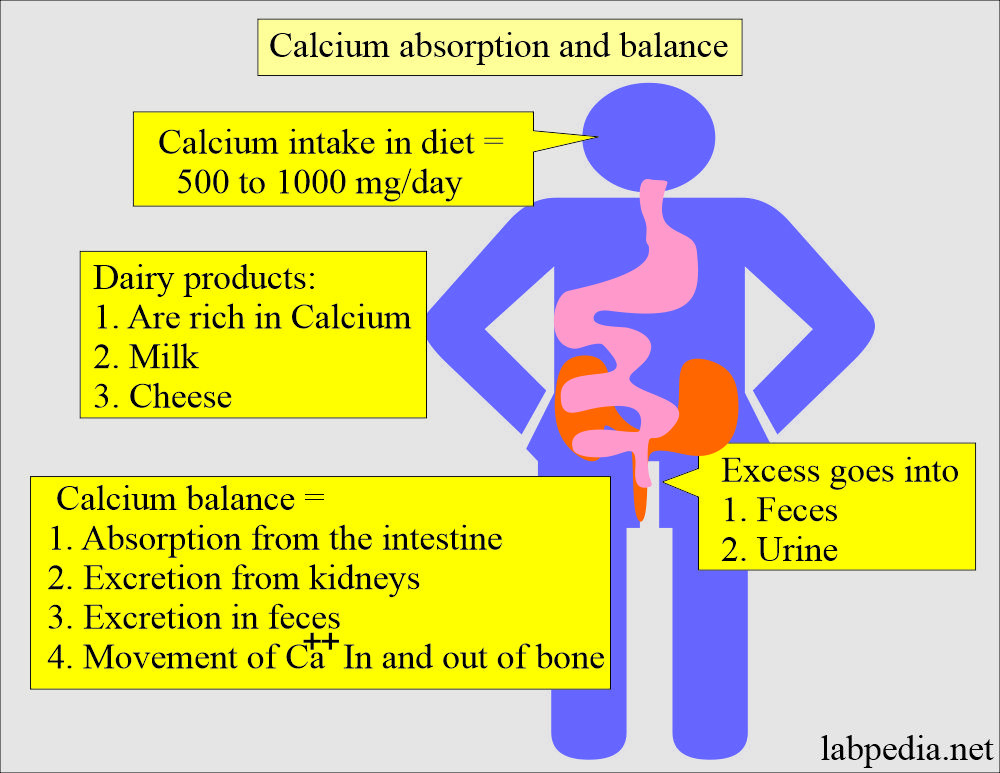
Intake of calcium and balance (Absorption and excretion):
- Most individuals ingest 500 to 1000 mg of calcium daily in their food.
- They excrete excess amounts in the feces and urine.
- Dairy products like milk and cheese are good sources of calcium.
- A large amount of dietary calcium is not absorbed because of the formation of insoluble calcium compounds like PO4, oxalate, phytate, and soap in the intestine and excreted in the feces.
- Calcium balance is maintained by:
- Absorption of calcium from the intestine.
- Excretion by the kidneys.
- Movement of calcium in and out of the bones.
- Calcium phosphate in the bone is not an inert substance.
- There is dynamic equilibrium with Ca++ and HPO4– of the body fluids by resorption and deposition.
Calcium functions:
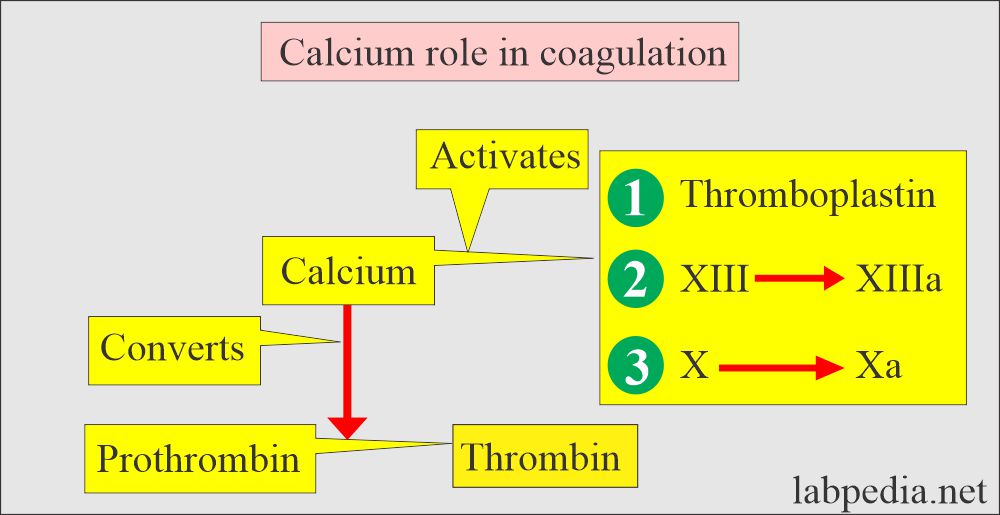
- Calcium is also needed to maintain metabolic processes like muscle contraction, the transmission of neural impulses, clotting of the blood, cardiac function, and inhibit cell destruction.
- Calcium stabilizes the plasma membranes and influences permeability and excitability.
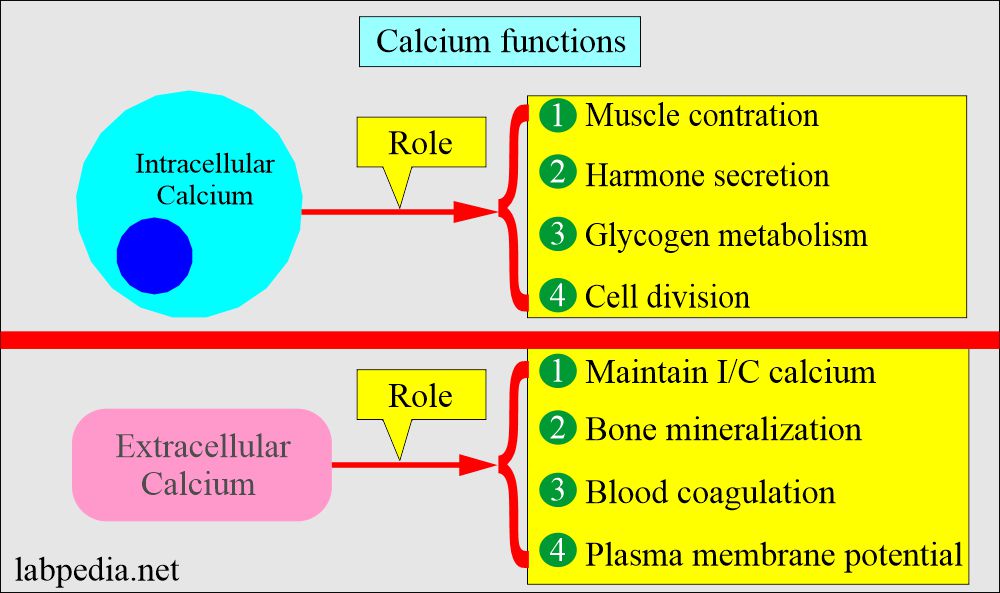
- The intracellular calcium functions are:
- Muscle contraction.
- Hormone secretion.
- Glycogen metabolism.
- Cell division.
- Activation of enzymes.
- Transfer of the ions across the cell membrane.
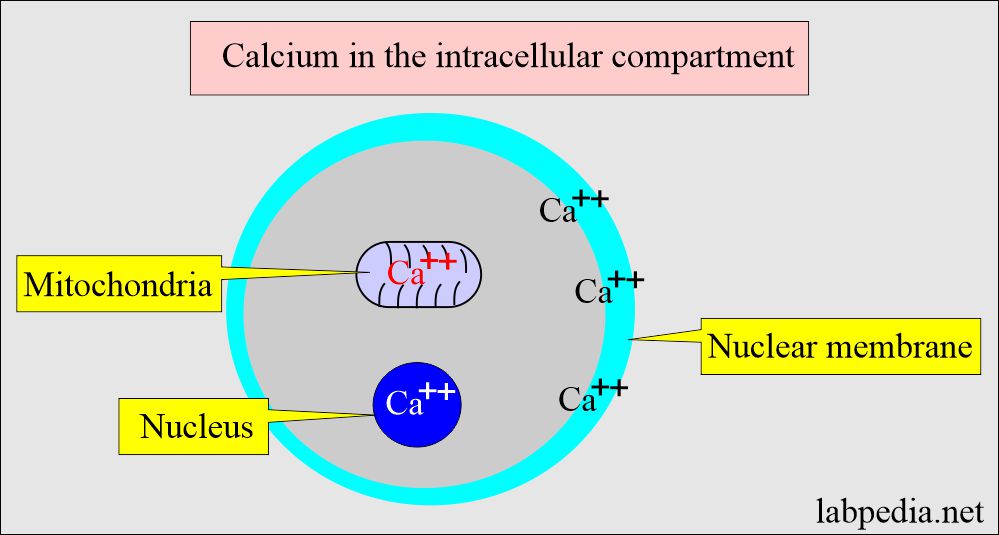
- The intracellular calcium is bound to:
- Protein in the cell membrane.
- Present in the mitochondria.
- Present in the nucleus.
- The extracellular calcium (extracellular calcium provides calcium ions) functions are:
- Bone Mineralization.
- Blood coagulation factors.
-
- Plasma membrane potential.
- Maintenance of intracellular calcium.
- Calcium decreases neuromuscular excitability.
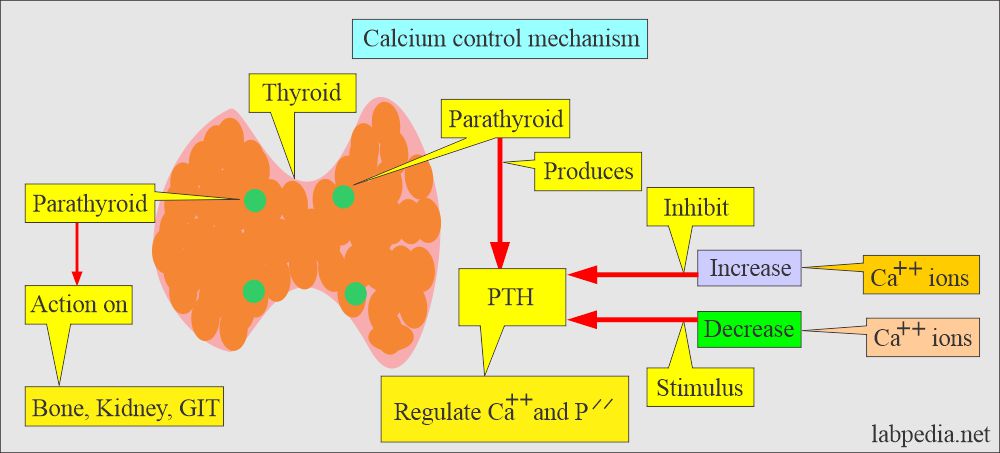
Calcium control mechanism:
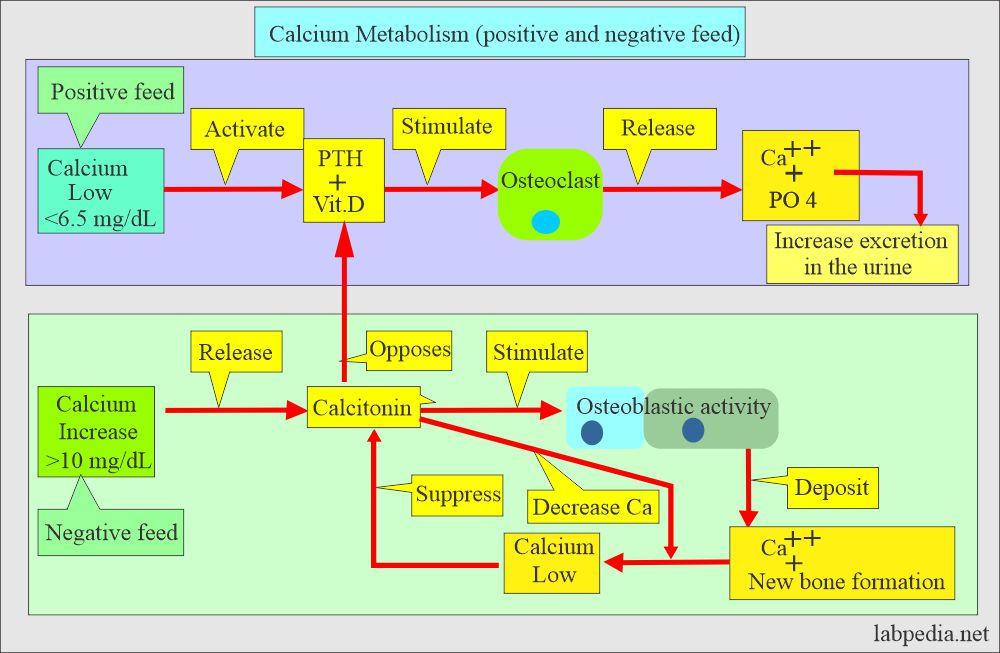
- Hypocalcemia:
- Normally the level of calcium in the blood is carefully controlled. When blood calcium levels get low is called hypocalcemia.
- The bones release calcium to bring them back to a normal blood level.
- Hypercalcemia:
- When blood calcium levels get high is called Hypercalcemia.
- The extra calcium is stored in the bones or passed out of the body in stool and urine.
- Body serum calcium levels are maintained:
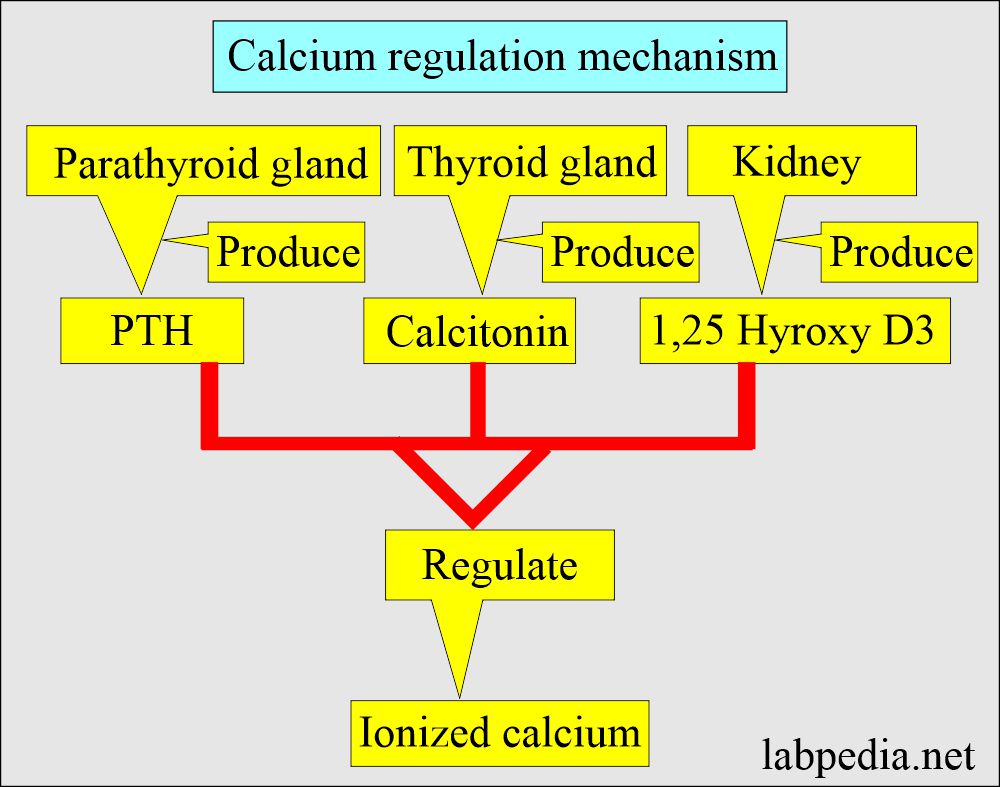
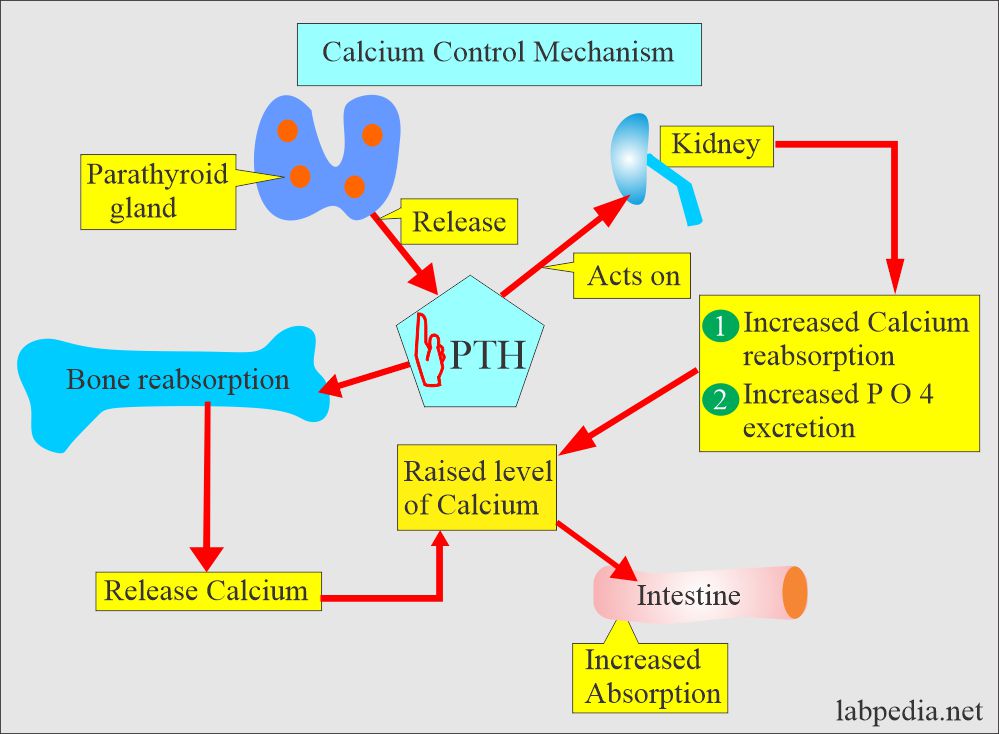
- By a parathyroid Hormone PTH (Parathyroid gland hormone, calcium regulating hormone).
- Calcitonin (produced by C or parafollicular thyroid cells) also plays a role in the control of serum calcium.
- The ectopic PTH-like hormone may be secreted by the tumors of the lung, breasts, and kidneys.
- PTH increases the serum calcium level:
- By increasing bone resorption.
- By mobilizing Calcium.
- PTH indirectly increases calcium absorption from the gastrointestinal tract by producing vit. D.
- PTH also increases the excretion of phosphate in the urine.
- Calcitonin decreases serum calcium and phosphate levels by inhibiting bone resorption.
- Decreasing calcium levels increases PTH, activating the calcium reservoir and releasing it into circulation.
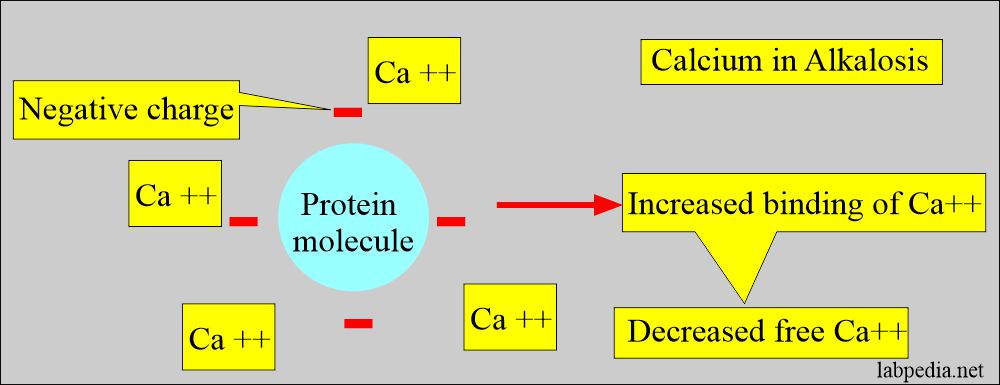
- Calcium binds to negative charge sites on the proteins, depending on the pH.
- Alkalosis, increased negative charge sites on proteins lead to increased calcium binding and decreased free calcium levels.
- In acidosis, negative charge sites are decreased, leading to increased free calcium.
- A patient with low serum albumin will have low serum calcium.
- So the serum albumin level may be estimated with the serum calcium level. Serum calcium level decreases to 0.8 mg with every decrease of 1 gram of albumin.
Effect of the albumin on serum calcium:
- A decrease in 1 gram/dL of serum albumin decreases total serum calcium by around 0.8 mg/dL.
- This is the average decrease, but this may change in different situations.
- Because no effect of serum albumin on ionized calcium is preferred.
Hypocalcemia:
Etiology of hypocalcemia:
- The most common cause is hypoalbuminemia.
- One gram/dL of albumin binds 0.8 mg/dL of calcium.
- So there may be decreased albumin-bound calcium.
- Or decrease in free calcium.
- One gram/dL of albumin binds 0.8 mg/dL of calcium.
- Chronic renal failure leads to hypocalcemia because:
- There is an increased loss of protein by the kidney in the urine.
- There is hyperphosphatemia.
- There is decreased serum 1,25(OH)2 D.
- There is skeletal resistance to PTH.
Signs and symptoms of hypocalcemia:
- Neuromuscular hyperexcitability.
- Like tetany.
- Paresthesia.
- Seizures.
- A rapid fall in calcium level may lead to hypotension.
- Acute symptomatic hypocalcemia may be associated with the following:
- Rapid remineralization of the bone after the surgery of primary hyperparathyroidism is called hungry bone syndrome.
- Acute pancreatitis.
Hypercalcemia:
Etiology of hypercalcemia:
- Hypercalcemia is due to an increase in the increased influx of calcium into the extracellular compartment from the:
- Skeletal system.
- Intestine.
- Kidney.
- Primary hyperparathyroidism is the most common cause in outdoor patients.
- Malignancy is the most common cause of hospitalized patients.
- The above two conditions constitute 90% to 95% of the causes of hypercalcemia.
- Also seen in 10% to 20% of the cases of cancers.
- Hypercalcemia (malignancy-associated) is seen in the following cancers:
- Myeloma = 30% (20% to 50%).
- Renal cell carcinoma = 11% (10% to 13%) due to ectopic production of PTH.
- Lung cancers = 10% (7% to 13%) due to ectopic production of PTH.
- Breast = 15% 7% to 23%) due to ectopic production of PTH.
- Non-Hodgkins lymphoma = 5% (3% to 13%).
- Leukemia = 3% (2% to 11%).
- Some lymphomas may produce 1,25(OH)2 D and cause hypercalcemia.
- Primary sarcoidosis shows hypercalcemia in 5% to 10% of the cases (1% to 62%).
Symptoms of hypercalcemia are:
- Anorexia.
- Lethargy.
- Nausea.
- Vomiting.
- Ultimately coma.
- Other patients may show:
- Nervousness.
- Excitability.
- Tetany.
To label hypercalcemia, one should have the following:
- Three separate raised levels of calcium.
- Serum or plasma calcium = >10.3 mg/dL
- Ionized calcium = >1.30 mmol/L.
A normal Calcium total:
Source 1
- Infant to one month = 7.0 to 11.5 mg/dL.
- One month to one year = 8.6 to 10.2 mg/ dL.
- Adult = 9 to 10.5 mg/dL.
- Its low level may lead to tetany.
Source 2
| Age | mg/dL |
| Cord blood | 8.2 to 11.2 |
| Premature | 6.2 to 11.0 |
| 0 to 10 days | 7.6 to 10.4 |
| 10 days to 2 years | 9.0 to 11.0 |
| 2 years to 12 years | 8.8 to 10.8 |
| 12 to 18 years | 8.4 to 10.2 |
| 18 to 60 years | 8.6 to 10 .0 |
| 60 to 90 years | 8.8 to 10.2 |
| >90 years | 8.2 to 9.6 |
- The conversion factor is x 0.25 for SI unit mmol/L
Hypercalcemia (plasma level = >10.5 mg/dL) may be seen in the following conditions :
- Hyperparathyroidism (primary and secondary).
- Hyperthyroidism.
- Acute and chronic renal failure.
- Following renal transplant.
- Metastatic bone tumor of lung, breast, and kidney.
- Bone metastasis is around 30% of the cases.
- 2% of Hodgkin’s lymphoma and non-Hodgkins lymphoma.
- Milk-alkali syndrome.
- Multiple myelomas.
- Paget’s disease.
- Osteomalacia with malabsorption.
- Aluminum-associated osteomalacia.
- Granulomatous diseases:
- Sarcoidosis (mostly uncommon).
- Tuberculosis.
- Leprosy.
- Uncommonly may be seen in:
- Mycosis.
- Berylliosis.
- Silicon granuloma.
- Crohn’s disease.
- Eosinophilic granuloma.
- Tumors produce a PTH-like substance.
- Vitamin D intoxication.
- Ectopic production of 1,25 -dihydroxy -vitamin-D3, e.g., from Hodgkin’s and Non-Hodgkin’s lymphoma.
- Vitamin A intoxication.
- Excessive calcium intake.
- Prolonged immobilization.
- Thiazide diuretics.
- Withdrawal of steroids.
- Most (80 to 90 %) of hypercalcemia cases are due to hyperparathyroidism or malignancy.
- Thyrotoxicosis in 20% to 40% of the cases is usually <14 mg/dL.
- Multiple endocrine neoplasias.
- Hypercalcemia is also seen in some of the neoplasms:
- Breast = 15% (7 to 23%).
- Lung = 10% (7 to 13%).
- Kidneys = 11% (10.5 to 13%).
- Colon = 5%.
- Cervix = 7%
- Non-Hodgkin’s lymphoma = 5% (3 to 13%).
- Leukemia = 3% (2 to 11.5%)
- Multiple myeloma = 30% (20 to 50%).
Causes of Hypercalcemia (>10.5 mg/dL):
| Clinical condition | Etiological causes |
| Primary hyperparathyroidism |
|
| Tertiary hyperparathyroidism |
|
| Drugs |
|
| Granulomatous diseases |
|
| Endocrine abnormality |
|
| cancers |
|
Hypocalcemia (plasma level = <8.5 mg/dL) may be seen in the following conditions :
- Hypoparathyroidism.
- Surgical.
- Idiopathic.
- Infiltration of the parathyroid gland by sarcoidosis, amyloidosis, hemochromatosis, and neoplasm.
- Hereditary conditions like DiGeorge syndrome.
- Pseudohypoparathyroidism is due to a lack of response to PTH.
- Malabsorption (inadequate absorption of nutrients from the intestinal tract).
- Hypoalbuminemia.
- Osteomalacia
- Pancreatitis
- Renal failure (chronic). Chronic renal failure with uremia and phosphate retention.
- It is also seen in Fanconi syndrome and renal tubular acidosis.
- Bone diseases like osteomalacia and rickets.
- Malabsortion of calcium and vitamin D.
- Insufficient ingestion of calcium, phosphorus, and vitamin D.
- Starvation.
- Liver disease (decreased albumin production).
- Obstructive jaundice.
- Hypomagnesemia.
- Hyperphosphatemia like phosphate enema and infusion.
- Drugs like:
- Cancer chemotherapy like cisplatin, cytosine arabinoside, and mithramycin.
- Antibiotics like ketoconazole, gentamicin, and pentamidine.
- Chronic use of anticonvulsant drugs like phenobarbitol and phenytoin.
- Use of calcitonin.
- Excessive use of fluoride (fluoride intoxication).
- Neonates of complicated pregnancies.
- Infants of diabetic mothers.
- Premature babies.
- In the case of osteoblastic tumor metastasis.
- In the case of cerebral injuries.
- Temporary hypocalcemia after subtotal thyroidectomy was seen in >40% of the patients, and >20% showed signs and symptoms.
Lab findings of hypocalcemia:
| Clinical conditions of hypocalcemia | PTH level | Serum PO4 | 1,25 hydroxy-D |
| Pseudohypoparathyroidism | Increased | Increased | Decreased |
| Hypoparathyroidism | Decreased | Increased | Decreased |
| 1,25-hydroxy-D resistance | Increased | Decreased | Increased |
| Vitamin D deficiency | Increased | Decreased | Low or normal |
Causes of hypocalcemia and lab findings:
| Lab parameters | Increased in the advised test | Decreased in the advised test |
| PTH level |
|
|
| Serum magnesium |
|
|
| Serum phosphate (Phosphorus) |
|
|
| Urine phosphate |
|
|
| Urine calcium level |
|
|
| Urine cAMP (cyclic adenosine monophosphate) |
|
|
The most common cause of hypocalcemia:
It may be seen in Hypoalbuminemia, in which the ionized fraction may be normal while the total calcium level is decreased due to the low percentage of calcium bound to albumin.
- A correction formula is needed as follows:
- Corrected calcium level = measured calcium — albumin g/dL + 4
Natural foods are a good source of calcium:
| Food | Quantity | Amount of calcium |
| Kale | one cup | 245 mg |
| Milk | one cup | 305 mg |
| Yogurt | 6 oz | 300 mg |
| Cheese | one oz | 224 mg |
| Dried figs | 8 whole figs | 107 mg |
| White Beans | one cup | 191 mg |
| Turnip greens | one cup | 195 mg |
| Black-eyed beans | 1/2 cup | 185 mg |
| Canned salmon | 1/2 cup | 232 mg |
| Orange juice | one cup | 500 mg |
| Orange | one medium | 65 mg |
| Sesame seed | one teaspoon | 88 mg |
| Almond | 1/2 cup dry roasted | 72 mg |
| Instant oatmeal | one cup | 187 mg |
| Soy milk | one cup | 300 mg |
| Firm Tofu | 1/2 cup | 861 mg |
| Broccoli | one cup | 62 mg |
Critical values:
- Acute hypo or hypercalcemia can be life-threatening
- > 14 mg/dL Hypercalcemia leads to come and cardiac arrest.
- < 4 mg/dL Hypocalcemia leads to tetany (another reference says <6.5 mg/dL).
Questions and answers:
Question 1: What is the calcium level which leads to tetany?
Question 2: What is the cause of hypercalcemia in renal cell carcinoma?