Anemia:- Part 7 – Hereditary Spherocytosis
Hereditary Spherocytosis
Sample for Hereditary Spherocytosis
- EDTA blood may be needed.
Definition of Hereditary Spherocytosis:
- Hereditary spherocytosis anemia is quite common and transmitted as an autosomal dominant trait in the caucasian population.
- Rarely it may be autosomal recessive.
- 75% of the cases are autosomal dominant inheritance patterns, and 25% are sporadic, and in most cases have a recessive inheritance.
- This is the most common hereditary hemolytic anemia in northern Europe, 1 in 5000.
- Inheritance is autosomal dominant in 75% of the cases.
- Cardinal features are:
- Chronic hemolysis.
- Jaundice.
- Splenomegaly.
Pathogenesis of Hereditary Spherocytosis:
- Molecular abnormality of the cytoskeletal proteins has been identified in some cases, these defects may be:
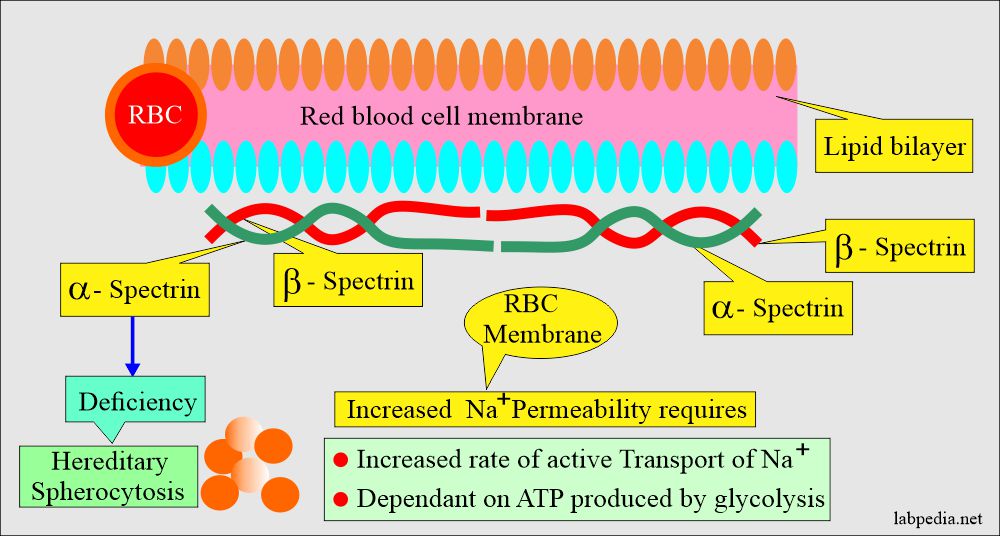
- Cytoskeletal proteins like band3 or protein4.2.
- Defect in the spectrin.
- Defect in the ankyrin.
- This basic defect is a partial deficiency of a protein called spectrin needed for the RBCs membrane’s cytoskeleton. Spectrin deficiency autosomal dominant pattern is the most common cause.
- The patients with the autosomal dominant pattern have 60% to 80% of normal spectrin, while recessive forms have 30% to 70% of the normal level.
- This is usually caused by the defect in the proteins involved in the vertical interaction between the membrane skeleton and the RBCs’ lipid bilayers.
- Spherocytes are not destroyed in the blood circulation, but these are sequestered and removed in the spleen.
- This condition will lead to splenomegaly.
- In these cases, splenectomy will cure the patients because the bone marrow can compensate.
The basic defect of Hereditary Spherocytosis:
- It is the loss of RBCs’ membrane caused by the release of parts of the lipid bilayer that is not supported by the skeleton, resulting in decreased surface area.
- This will produce RBCs with the lowest surface-area-to-volume ratio called spherocytes.
- The marrow produces normal biconcave RBCs, but these lose membrane and become more spherical, where there is a loss of surface area compared to volume.
- These RBCs can not change their shape easily, so they can not move through the microcirculation, particularly through the spleen reticuloendothelial system, where they will die permanently.
- In one of the articles, hereditary spherocytosis is classified into four subtypes.
- Minor HS.
- Moderate HS.
- Moderate to severe HS.
- Severe HS.
| Clinical parameters | Minor HS | Moderate HS | Moderate to severe | Severe HS |
| Hemoglobin | Normal | >80% | 60% to 80% | <60% |
| Reticulocytes | <6% | 6% to 10% | >10% | >10% |
| Peripheral blood smear | Few spherocytes | Spherocytes | Spherocytes | Microspherocytes and poikilocytosis |
| Osmotic fragility at 37 °C | Increased | Increased | Increased | Increased |
| Splenectomy | Rarely needed | Depending upon certain cases | Necessary >5 years old | necessary > 2 to 3 years old |
| Inheritance | Autosomal dominant | Autosomal dominant, de novo | Autosomal dominant, de novo | Autosomal recessive |
Differential diagnoses of hereditary spherocytosis:
- It may be seen in the ABO transfusion reaction.
- These are seen in widespread malignancy.
- These are seen in Clostridium welchii septicemia.
- Seen in severe burns.
- Sometimes seen in autoimmune hemolytic anemia.
Signs and symptoms of Hereditary Spherocytosis:
- Hereditary spherocytosis is quite a common cause of hemolytic anemias, more than hemoglobinopathies and G-6-PD deficiency.
- There is hemolysis, and is present in >90% of the cases.
- 50% to 60% of the cases are compensated by the hyperplasia of the bone marrow, and no anemia may be seen except in crises.
- The prominent features of hereditary spherocytosis:
- Chronic hemolysis.
- Jaundice is found in 50% of the cases and is intermittent.
- Splenomegaly was found in 50% of the cases of young children. It is found in 80% of older children and adults (In the literature reported as 72% to 95%).
- Anemia is mild and usually is unnoticed and diagnosed at an adult age.
- Anemia may be present at any age, from infancy to old age.
- Anemia may be mild to moderate, and Hb is >8 g/dL.
- There is an increased tendency for the bilirubin levels.
- The jaundice is fluctuating. It is marked if it is associated with Gilbert’s syndrome.
- Chronic hemolysis leads to pigment. Gallstones are quite common in these patients. In old patients, 55% to 75% develop gallstones, which may be seen even in young children.
- In the spleen, these cells, by removing the membrane, change into microspherocytes and are ultimately sequestered, leading to splenomegaly.
- Spenemegally is quite common in these patients.
- These patients ultimately develop anemia, splenomegaly, and ulcer legs.
- Aplastic crises are usually seen in patients with parvovirus infection. This will lead to the severity of anemia.
- Megaloblastic anemia is due to folate depletion due to the bone marrow’s overactivity.
- Autohemolysis is increased, and this can be corrected by glucose.
- Gallstones develop in 55% to 75% of the patients by the time of old age and may be seen in even young children.
Lab. findings of Hereditary Spherocytosis:
- There is evidence of hemolysis in 90% of the cases.
- 50% to 60% of the patients can compensate for the bone marrow hyperplasia and do not show anemia except in crises.
- There is mild to moderate anemia (8 to 12 G/dL).
- MCV is normal, slightly low, or even high due to reticulocytosis.
- MCHC is high.
- MCV and MCHC are within the normal range in about 80% of the cases; for the rest, in 20%, this value may be increased or decreased.
- MCHC is usually in the normal range but increases in 20% to 50% of cases.
- Increased MCHC value indicates congenital spherocytosis.
- Increased reticulocytes, 5% to 7% (another reference 5% to 20%). Reticulocytes are raised in 98% of the cases, and the mean count is 9%.
- Osmotic fragility is very high, and this is a confirmatory test.
- This test needs 24 hours of incubation at 37 °C to become prominent, particularly in newborns.
- The procedure of the osmotic fragility test:
- Keep the normal saline with decreasing dilution in different tubes.
- In diluted saline, normal RBCs start hemolysis.
- Spherocytes are more susceptible to hypotonic saline for hemolysis than normal RBCs.
- Spherocytes start hemolysis at a concentration above the normal range.
- Bilirubin slightly increased. 50% of the patients develop jaundice which is intermittent.
- There is detectable urine urobilinogen.
- Raised LDH level.
- Urine urobilinogen is increased.
- Haptoglobin low.
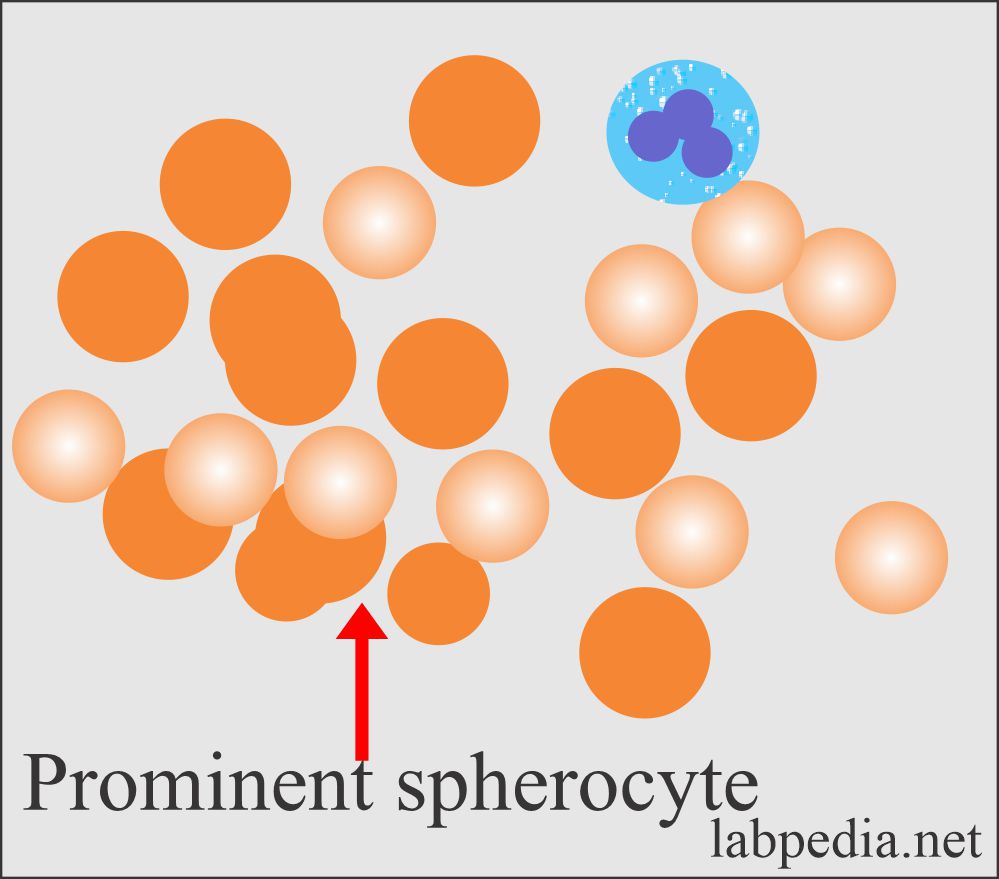
- Peripheral blood shows prominent spherocytes. The classic spherocyte is smaller or just near the size of normal RBCs. It is round and does not show a central clear area.
- Smaller spherocytes are called microspherocytes and are seen in other types of hemolytic anemia, e.g., hemolytic transfusion reaction.
- 20% to 25% of the cases show few spherocytes.
- Spherocytes are densely stained with a smaller diameter than the normal RBCs.
- Spherocytes are uniform round RBCs with more intensely staining hemoglobin and no central pallor.
-
- There is increased polychromatophilia.
- Reticulocytes are seen.
- Bone marrow shows erythroid hyperplasia.
- Direct Coomb’s test is negative.
- The differential diagnosis for immune-mediated hemolysis:
- In HS, a direct antiglobulin test is negative.
- In immune-mediated hemolysis, the MCV is low.
Complications of Hereditary Spherocytosis:
- Hemolytic crises are usually associated with viral infections. The Hb level decrease is not severe.
- The Hb level drops in this situation, and WBCs, RBCs, and platelet production are stopped.
- Aplastic crises usually accompany abdominal pain and fever, lasting 6 to 14 days.
- Bone marrow production of WBCs and RBCs, and platelets are stopped, and there is low hemoglobin.
- Megaloblastic deficiency leads to megaloblastic changes, and it has been in congenital spherocytosis.
- With increasing age, there is a risk of pigmented gallstones which may be seen in 50% of the cases.
Differential diagnosis of Hereditary spherocytosis anemia:
- This hereditary spherocytic anemia needs to differentiate from:
- Immune hemolytic anemia.
- G6PD deficiency.
- Thermal injury.
- Toxins due to infection of Clostridium.
- Snake venom.
- Bee and spider venom.
Treatment of Hereditary Spherocytosis :
- For the treatment of symptomatic patients, splenectomy is the choice.
- Because increased bone marrow production of RBCs can compensate for the presence of spherocytes, which have a shorter life span than the normal RBCs.
- The patient should be kept on folic acid prophylactically to prevent aplastic crises.
Question 1: What is the main defect in the RBC membrane of hereditary spherocytosis.
Question 2: What is the best test for confirmation of hereditary spherocytosis.