Anemia:- Part 4 – Thalassemia, α-thalassemia and β-thalassemia, Workup and Diagnosis
Thalassemia
Sample for Thalassemia
- Venous blood is needed.
- Prepare a fresh peripheral blood smear.
Definition of thalassemia:
- Thalassemia has inherited hemoglobinopathies resulting from the decreased production rate of one or more globin chains of hemoglobin. Or
- These are a heterogeneous group of genetic disorders resulting from the decreased synthesis of α or β chains of hemoglobin.
The decreased hemoglobin synthesis leads to the following:
- Decreased hemoglobin in the RBCs.
- Hypochromasia.
- Microcytosis.
- Variable degree of hemolysis.
- Also called Cooley’s anemia.
History of Thalassemia:
- Thalassemia derives from the combination of the Greek word Thalassa means sea, and Haima means blood.
- This was known as Mediterranean anemia because of the most common occurrence in the Mediterranean population.
- This is characterized by a decreased rate of production of globin chains. These are classified according to the globin which is involved.
- The consequence is defective globin chain production.
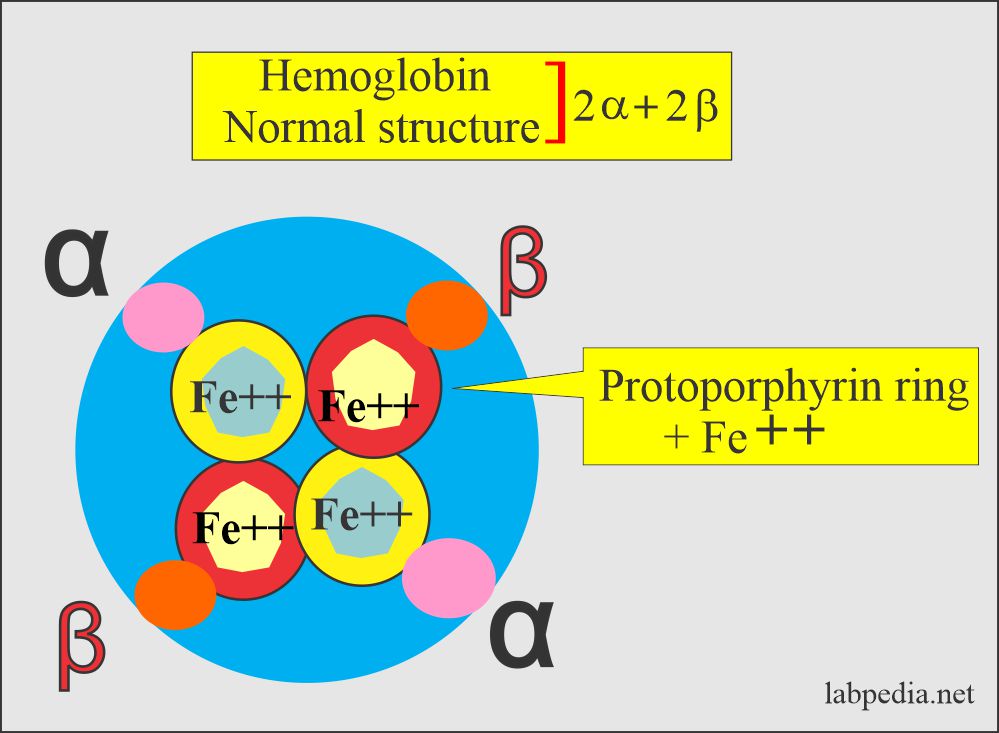
To understand thalassemia, we need to discuss and understand the structure of the Hemoglobin:
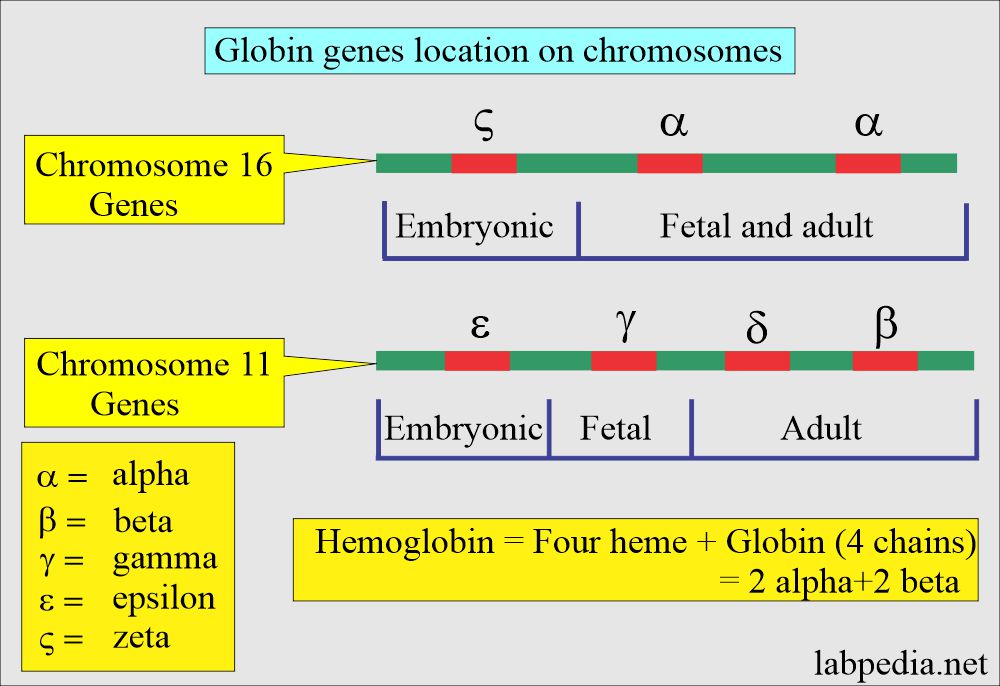
- The normal globin, which is part of the hemoglobin, consists of 2 alpha and 2 beta chains.
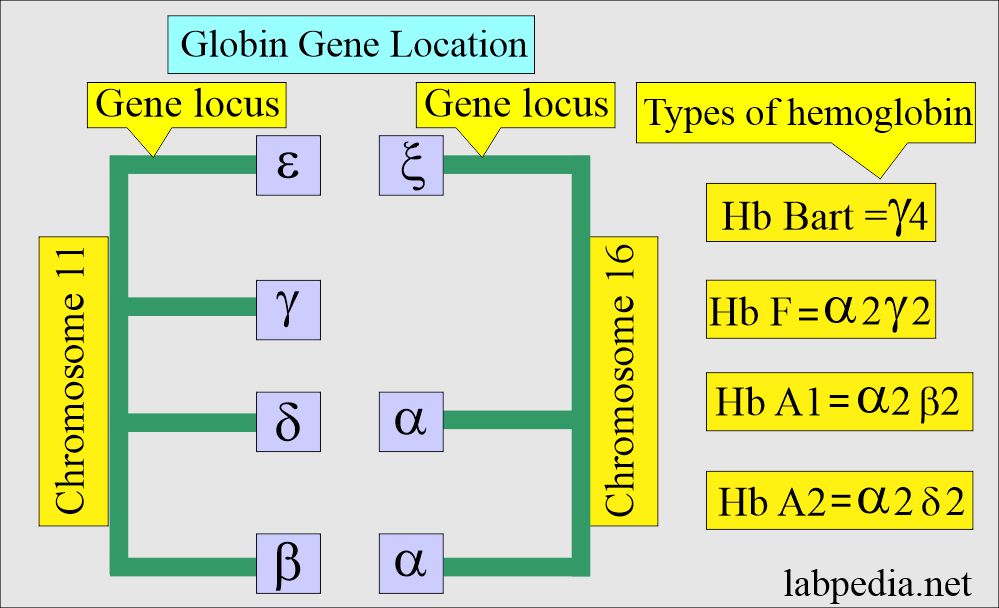
Various types of hemoglobin and their structures:
| Type of hemoglobin | Genotype of hemoglobin | Hemoglobin presence |
| Hb A | α2β2 | This is the main adult Hb |
| Hb A2 | α2/δ2 | This is present in a small amount |
| Hb F | α2/γ2 | Main fetal Hb in late stages |
| Hb gower1 | ζ2/ε2 | This Hb is present in the early life of the fetus |
| Hb gower2 | α2/ε2 | This Hb is present in a small amount in the early fetal life |
| Hb portland | ζ2γ2 | It is seen in embryos. |
| Hb H | β4 | It is seen in α-thalassemia |
| Hb Bart’s | γ4 | It is seen in α-thalassemia |
- Each pair is inherited from each parent.
- So one α/β gene is inherited from the father and the other α/β pair from the mother.
- In thalassemia, a gene may involve either α or β chains.
- In the majority of the patients, β-chain is involved.
- HbA1 has 2 α and 2 β-chains.
- HbA2 has 2 α and 2 δ-chains.
- HbF has 2 α and 2 γ-chains.
- All these hemoglobin HbA1, HbA2, and HbF are present in the adult RBCs.
- HbA2 and HbF are present in trace amounts.
Genetic codes and normal hemoglobin:
- The genes located on chromosome 11 are γ, δ, ε, and β-chains.
- While on chromosome 16, there are 2 α and ζ loci.
- β-thalassemia:
- Only one of the β-chain is involved in heterozygous conditions, called β-thalassemia minor.
- In homozygous conditions, both β-chains are involved, called β-thalassemia major.
- α-thalassemia:
- α-chain involvement is more complicated.
- Because there are 2 α-gene loci on chromosome 16, while the β-gene is only one locus on chromosome 11.
- In silent carriers of α-thalassemia, only one of the 4 α-genes (1/4) is absent (deleted or abnormal).
- In α-thalassemia minor, 2 of the 4 α-genes (2/4) are affected. There may be deletion or abnormality in both gene loci.
- In α-thalassemia-1, which is more common in Asians.
- In α-thalassemia-2, which is more common in Africans and Medittranians.
- HbH disease occurs because of the deletion or inactivation of the three gene loci (3/4). So all 4 globin chains are β-chains.
- Hb Bart’s disease is a more serious disease; it occurs when all the 4 α-genes (0/4) are deleted or inactivated. There are all 4 γ-globins.
Mechanism of Thalassemia:
- Thalassemia syndrome may occur because of the abnormality of the following:
- Coding sequence.
- Transcription.
- Processing or defects in gene translation leads to thalassemia.
Classification of the Thalassemia:
- The older classification was classifying thalassemia based on the severity of the disease as follows:
Thalassemia major:
- α-globin genes are absent (0= –/–).
- Hb Bart’s at birth is 75%.
- MCV = 110 to 120 fl.
- MCH is greatly decreased.
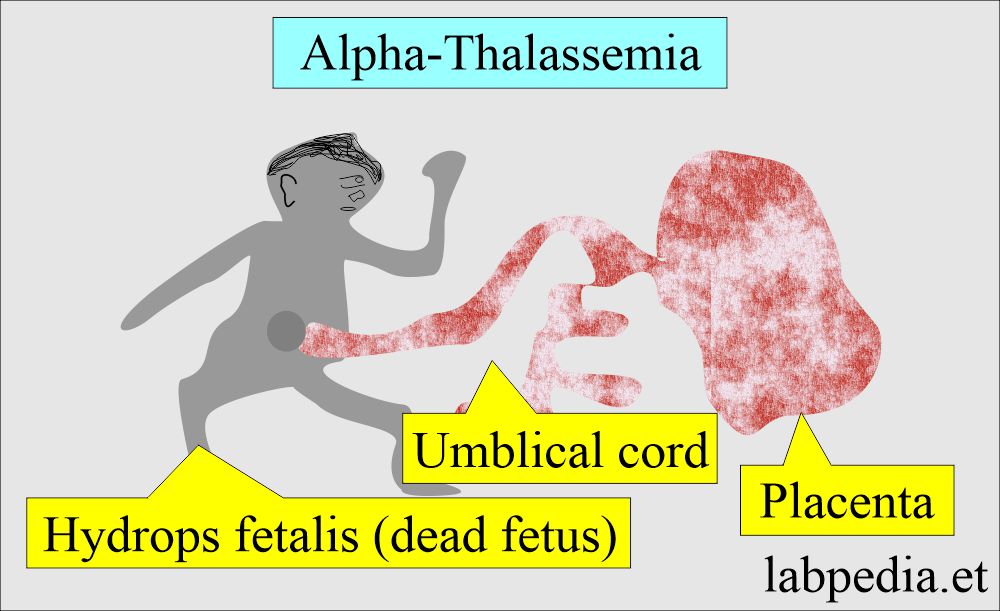
- This is also called hydrops fetalis.
- Signs and symptoms:
- The complete absence of the α-globin genes in fetal life leads to intrauterine death of the fetus due to severe hypoxemia.
- This is due to Hb Bart’s, which has a high affinity for oxygen and prevents the release of O2 to the tissues.
- At birth, no S/S.
- Infants during 3 to 6 months show pallor, yellow skin, and sclera.
- Infants from 6 to 12 months show severe anemia and bone abnormalities and can’t thrive.
- There are life-threatening complications.
- There is splenomegaly or hepatomegaly.
- These patients will have frequent infections.
- There is a tendency for bleeding, like a nosebleed.
- These patients have a small body, but the large head is a characteristic feature.
- These infants may be mentally retarded.
- The complete absence of the α-globin genes in fetal life leads to intrauterine death of the fetus due to severe hypoxemia.
Thalassemia Intermedia:
- There is some degree of anemia, jaundice, and splenomegaly.
- There are signs of hemosiderosis, such as hemoptysis.
- There is iron deficiency anemia.
Thalassemia minor:
- One globin gene is absent (-α/αα).
- These are the silent carrier.
- There are usually no symptoms.
- There is mild anemia.
- MCV is normal to slightly decreased.
- HbH small amount of 1% to 2% may be present at birth. This will disappear later on.
- Often these patients are overlooked.
Thalassemia minima:
- It is a mild disease.
- It is a silent carrier of the β-thalassemia trait.
- Anemia is not evident.
- HbA2 = normal or slightly increased. HbF is increased.
- Normal RBC morphology and Hb electrophoresis.
The second classification of thalassemia:
- It is based on the genetic makeup of the hemoglobin, and it is divided into:
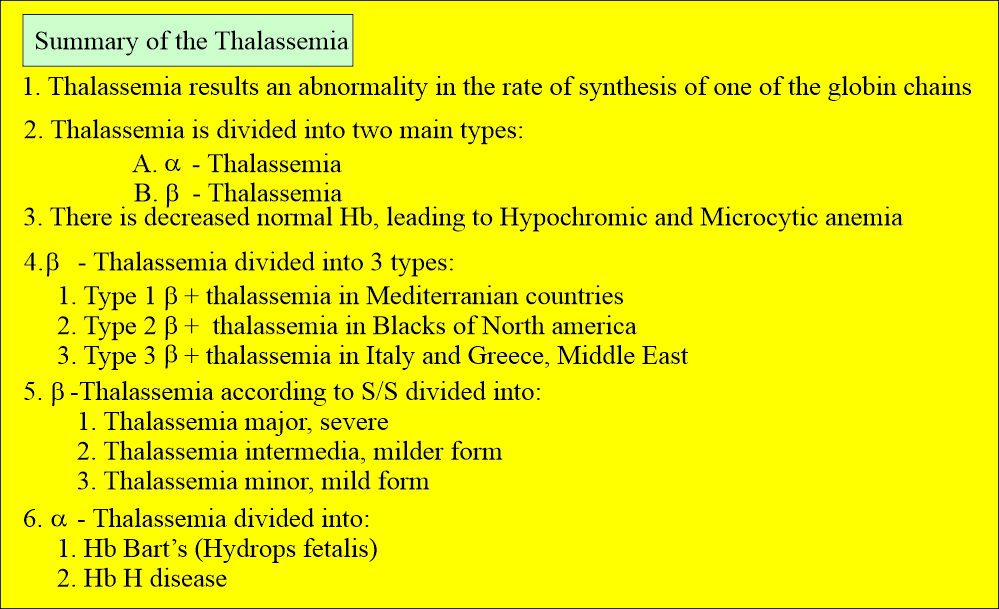
- α-Thalassemia.
- β-Thalassemia
Alpha- thalassemia (α-thalassemia)
- α-thalassemia is a group of genetic disorders with defective α-chain synthesis.
- Chromosome 16 carries 2 α genes, and the total number of α-gene is 4.
- Severity depends upon the patient’s affected number of genes one, two, three, or four.
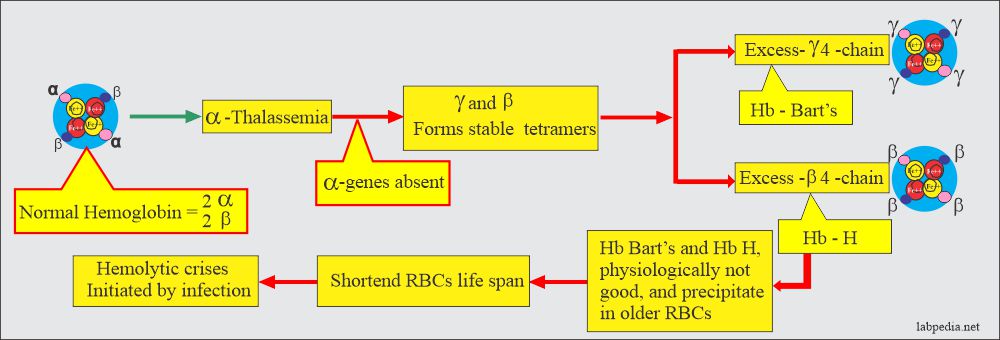
- Decreased synthesis of α-chain will decrease the synthesis of HbA, HbF, and HbA2 because these chains have α-chains; the net result will be an excess of β-chains and γ-chains. These chains may polymerize into tetrameric forms γ4 called Hb Bart’s and β4 called HbH.
- These abnormal Hb Bart’s and HbH are the characteristics of α-thalassemia.
- Usually manifested immediately after birth or even in utero because the α-gene is activated early in fetal life.
- α-thalassemia has a wide range of clinical presentations.
- Chromosome 16 carries 2 α genes; the total will be 4 α-genes (each pair from the parents). This will vary the severity of the diseases, depending upon one: two, three, or four genes affected in one patient.
- Another feature of α-thalassemia is that decreased or absent α-gene production will result in more than γ-chain during fetal life and at birth and excess of β-chain later on. This will lead to stable tetramers, γ4 (Hb Bart’s) and β4 (Hb H). Hemoglobin Bart’s and H precipitate in the older RBCs. These may lead to hemolytic crises by infection. This abnormal hemoglobin can be detected by electrophoresis.
α-thalassemia minor:
- These are silent carriers.
- There is decreased production of the α-chain (α+-α / ββ).
- One α-globin gene is affected = -α/αα.
- These are the silent carrier, and there is no marked anemia.
- MCV will be normal to decrease slightly.
- Hb H (1% to 2%) is present at birth and disappears later.
α-thalassemia trait:
- It has 2 α-globin genes affected = α-/α- or αα/–.
- RBCs show microcytosis and hypochromic anemia.
- MCV is <70fl.
- There is mild anemia.
- Serum electrophoresis showed 5% to 10% Hb H (4 β) at birth, which will disappear later.
α-thalassemia major:
- It is Hb H disease.
- Three α-globin genes are affected = α-/–.
- There is microcytic hypochromic anemia.
- MCV is <70 fl.
- Serum electrophoresis showed predominantly Hb Bart’s, consisting of 4 gamma chains at birth.
- There is a gradual shift to Hb H 5% to 30% over the first few months of life.
Alpha-thalassemia Characteristic features:
| Clinical features | Genotype structure | Electrophoresis pattern | Peripheral blood smear |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
α-thalassemia classification and characteristic features:
| The genotype of α-thalassemia | Severity of anemia | Hb at birth | Hb at adult | α-chain deletion | Clinical outcome |
| α-thalassemia carrier |
Normal picture |
|
Asymptomatic | ||
| α-thalassemia 1 trait | Hypochromic ± | Hb Bart’ 5% to 10% | Hb A, A2, and F |
|
|
| α-thalassemia 1/α-thalassemia 1 (Hydrops) | Hypochromic +++ | Hb Bart’s 80% | Trace of HbH and Portland |
|
Incompatible with life |
| α-thalassemia 2/trait | Hypochromic ± | Hb Bart’s 1% to 2% | HbA, A2, and F |
|
|
| α-thalassemia 1/α-thalassemia 2 (HbH) | Hypochromic ± and inclusions |
Hb Bart’s 1% to 15%
|
HbB 4% to 30% |
|
Clinical features of alpha-thalassemia:
- In case of loss of all 4 α-genes, life is incompatible and leads to the fetus’s death (hydrops fetalis).
- Microcytic hypochromic anemia with splenomegaly. This is known as Hb H disease because of the presence of Hb H (β4). Can find this Hb on electrophoresis.
- In fetal life, Hb Bart’s is seen.
- α-Thalassemia trait is caused by the loss of one or two α-genes that are not usually associated with anemia, but MCV and MCH are low.
Beta-thalassemia (β-thalassemia):
Beta-thalassemia major:
- This is also called Cooley’s anemia and is the homozygous state of β-thalassemia
- It consists of 2 α chains and 2 γ-chains.
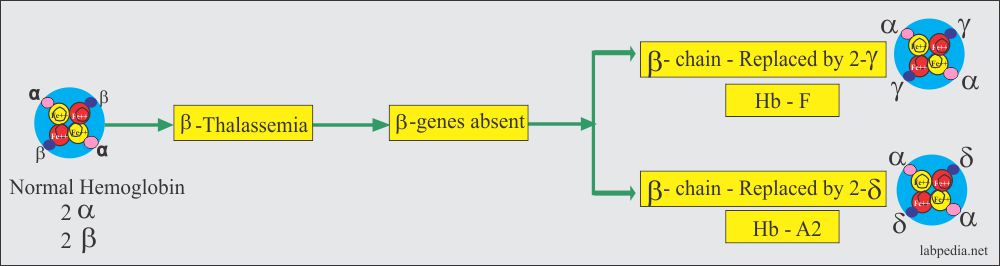
- There is decreased production of the β-chain (α2 / β0 β0). A globin gene mutation causes partial β-gene or total β-gene chain loss.
- The number of genes affected, partial or complete, will determine the severity of the disease.
- There is an increase in the production of γ-chains and δ-chains, resulting in increased Hb F and Hb A2 levels.
- The β chain is replaced by the 2-γ chain, which will form Hb F; the other is replaced by δ-chains, which will form Hb A2.
- These are usually homozygous (β0β0):
- β0β0-thalassemia is a more severe variant. No β-chains are synthesized.
- No Hb A found on electrophoresis.
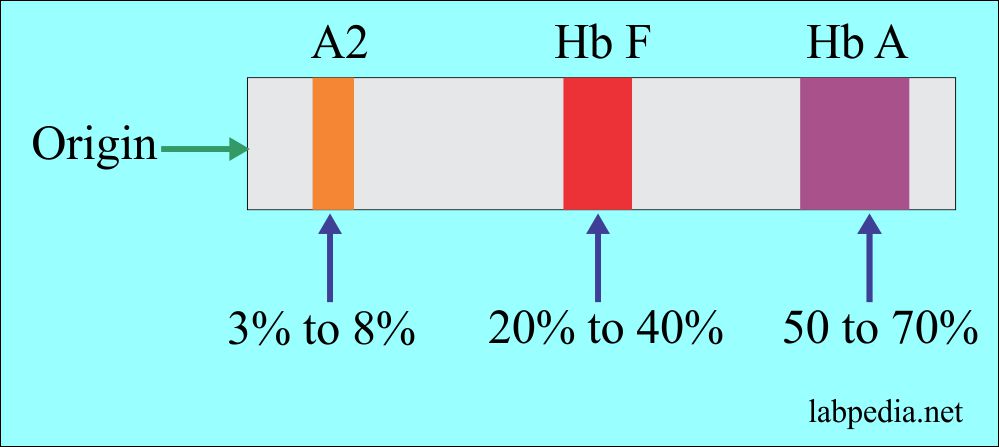
- Only HbF (>90%) and HbA2 (3% to 8%) are found.
- This is also called Cooley anemia.
- There is marked microcytosis and hypochromasia.
- MCV is <70 fl, and Hb is 2 to 3 g/dL.
- There is hepatosplenomegaly, bony deformities, and failure to thrive as an infant.
- These patients are dependent upon blood transfusion.
Beta – thalassemia minor:
- where a single β-gene is affected (β0/β).
- There is mild anemia Hb 9 to 11 g/dL or no anemia.
- Normal to increased RBC count.
- RBCs are microcytes, MCV 60 to 70 fl.
- Electrophoresis shows a mild increase in Hb F and Hb A2 (3% to 8%).
Beta – thalassemia intermedia:
- It is most commonly caused by partial deletion of β0 of both beta genes.
- These are homozygous (β+β+) genes.
- It will give a wide spectrum of the disease with moderate to severe anemia, and Hb will be 6 to 10 g/dL.
- There are growth retardation and bony abnormalities.
- This usually occurs later than the major thalassemia type.
- Electrophoresis shows Hb F 20% to 40% and increased Hb A2, 3% to 8%.
Another classification:
- β0+ shows a complete absence of the production of the beta chains.
- This is found in the Mediterranean, particularly in Northern Italy, Greece, Algeria, and Saudi Arabia. And Southeast Asia.
- β+-thalassemia is less severe.
- There are three groups of this gene rearrangement.
- 1β+ thalassemia gene produces less amount of the beta-chain, around 10% of normal production. This group is found throughout the Mediterranean, middle east, Indian subcontinent, and Southeast Asia.
- The 2β+ thalassemia gene produces more beta-chain, around 50% of the normal population. This is found in the blacks of North America and West Africa.
- The 3β+thalassemia gene produces even more beta chains, leading to milder disease. It is found particularly in Italy, Greece, and the Middle East.
- Severe thalassemia is called thalassemia major.
- Sever hypochromic and microcytic anemia develops during the first year of life.
- Hemoglobin is <7 g/dL and consists mostly of HbF and HbA2.
- Homozygous type 2 and 3 beta+ causes a milder form of thalassemia called thalassemia intermedia.
- The heterozygous beta-thalassemia gene causes a milder form of anemia.
- This also shows mild hypochromasia and microcytosis called thalassemia minor.
- The minor group may show delta-chain abnormality.
- The heterozygous beta-thalassemia gene causes a milder form of anemia.
- Beta-delta thalassemia (δβ) is another rare form of thalassemia characterized by the combined defect in δ and β chain synthesis.
- This group may have a normal level of Hb A2 and usually a high level of Hb F in the heterozygote and absent Hb A and A2 in the homozygote.
- δβ-thalassemia can be divided into two groups according to Hb F found:
- If γ-gene is active, then that group is called GγAγδβ thalassemia.
- Another type that has inactive γ, δ, and β genes is called Gγδβ thalassemia.
Beta-thalassemia syndrome shows:
| Type of β-thalassemia | Genotype of β-thalassemia | Characteristic features |
|
|
|
|
|
|
Clinical features of beta-thalassemia:
- In beta-thalassemia major, there is severe anemia, which appears 3 to 6 months after birth.
- The liver and spleen enlargement is due to increased destruction of the RBCs, intramedullary hemopoiesis, and iron overload.
- Splenomegaly needs more blood and increases RBC destruction and pooling.
- Bone marrow hyperplasia in thalassemia leads to a thalassemic face. There is thinning of the cortex of the bones, which may lead to bone fractures.
- X-rays may show the bossing of the skull and typically a hair-on-end appearance.
- Lab findings of beta-thalassemia:
- Low Hemoglobin.
- The peripheral blood smear shows Hypochromic and microcytic anemia.
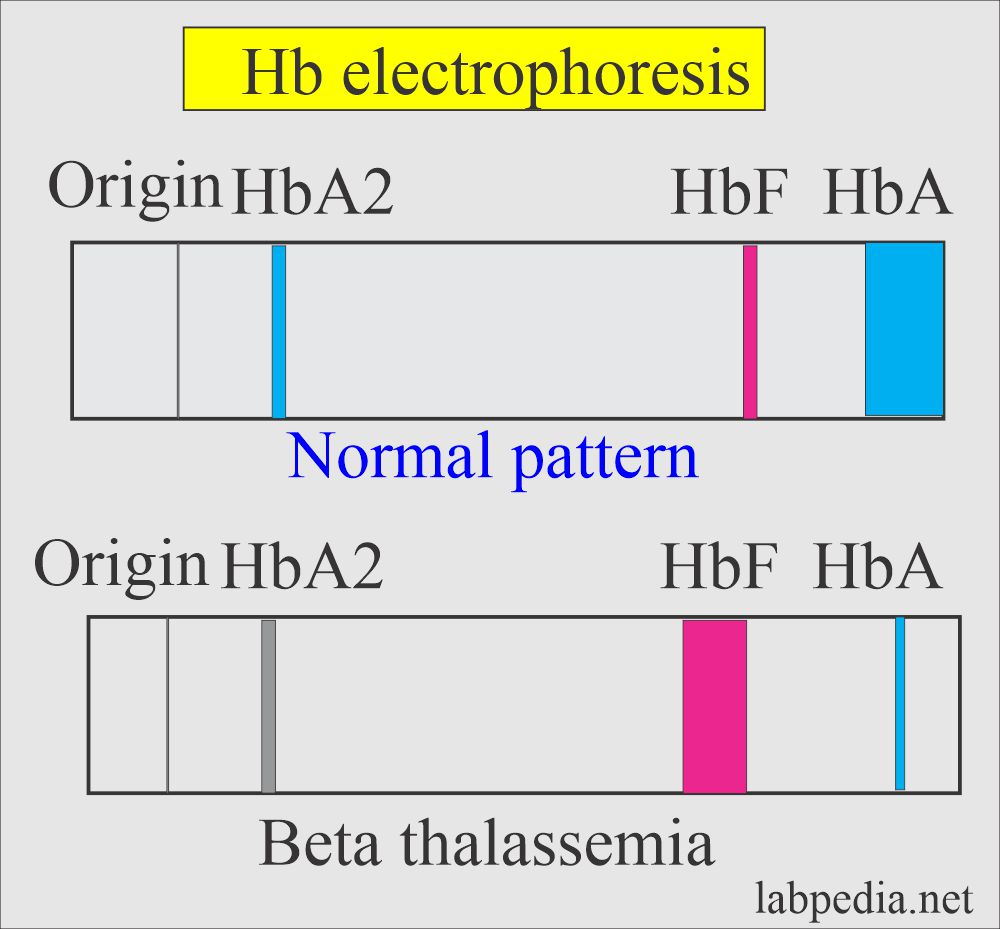
- Hb electrophoresis confirms the diagnosis by the near absence of the decreased level of Hb A.
Hemoglobin on electrophoresis is different in different types of thalassemia.
| Patient | % of the type of Hemoglobin |
|
|
|
|
|
|
|
|
|
|
Summary of Thalassemia types:
- α-Thalassemia trait is due to double gene deletion.
- There are microcytes and hypochromasia.
- α-Thalassemia disease is due to three gene deletions.
- Target cells, ovalocytes, microcytes, and Hb H are included in the RBCs.
- β-Thalassemia in heterozygotes, and there is β gene deletion alone or combined with the δ gene.
- There are microcytes, target cells, elliptocytes, and basophilic stippling.
- β-Thalassemia in homozygotes, and there is β gene deletion alone or in combination with the δ gene.
- It is marked as hypochromasia with polychromatic rims. There are target cells, ovalocytes, basophilic stippling, and HbH crystals.
Beta-thalassemia differential diagnosis:
| Characteristics | Homozygous β-thalassemia | Heterozygous β-thalassemia |
| Hemoglobin | 2 to 5 g/dL | 9 to 11 g/dL |
| RBC morphology |
|
|
| Reticulocytes count | ≥15% | It is mildly elevated. |
| Platelets |
|
|
| WBC count |
|
|
| Bone marrow |
|
|
| Hb A2 | Variable | 3.5 to 7% |
| Hb F | 10 to 90% |
|
| Storage iron |
|
|
Treatment of thalassemia major:
- These patients survive by blood transfusion. It is tried to maintain hemoglobin levels over 10 g/dL.
- It usually requires 2 to 3 units every 4 to 6 weeks.
- Fresh blood, filtered to remove white blood cells, gives the best RBCs survival and fewer reactions.
- 500 mL of blood contains 250mg of iron.
- Regularly give the folic acid 5 mg/day.
- Iron supplements are contraindicated.
- Iron overload is a complication that needs chelating therapy to control iron overload.
- Deferoxamine is the most common drug used for the chelation of iron.
- This can be given 1 to 2 mg with each unit of the blood.
- Give 40 mg/kg subcutaneously over 8 to 12 hours, 5 to 7 days weekly.
- This should be started in infants after 10- to 15 units of blood transfusion.
- Excess iron causes skin pigmentation and damages the heart.
- Assessment of the iron status advises:
- Serum ferritin.
- Serum iron.
- % saturation of transferrin.
- Serum non-transferrin bound iron.
- Assessment of the iron status advises:
- Bone marrow biopsy for reticuloendothelial stores by Perl’s stain.
- Liver biopsy for parenchymal and reticuloendothelial stores.
- Assessment of the tissue damage caused by the iron overload:
- For heart damage caused by iron, advice:
- X-ray chest.
- ECG, 24-hour monitoring.
- Echocardiography.
- Radionuclide scans to check left ventricular ejection.
- For liver damage by the iron advice:
- LFT.
- Liver biopsy.
- CT scan or MRI.
- For endocrine glands damage caused by iron, advice:
- Glucose tolerance test.
- Pituitary gonadotropin release test.
- Growth hormone assay.
- Radiology of the bones.
- Isotope bone density study.
- Functional tests of the thyroid, parathyroid, adrenal, and gonadal glands.
- Vitamin C 200 mg/day. This will help in the excretion of iron produced by deferoxamine.
- Immunization against the Hepatitis B virus.
- Allogenic bone marrow transplantation will give a permanent cure.
- Infections are quite common in these patients, and need treatment by antibiotics.
- Treatment for β-thalassemia:
- This is supportive treatment.
- Antibiotics for infections.
- Folic acid supplement.
- Transfusion of packed RBCs to raise the hemoglobin.
- Splenectomy may be advised.
- Bone marrow transplantation may be done.
Alpha-thalassemia needs:
- Blood transfusion.
- In utero, can give blood transfusion in case of hydrops fetalis.
Screening and diagnosis of the thalassemia patient:
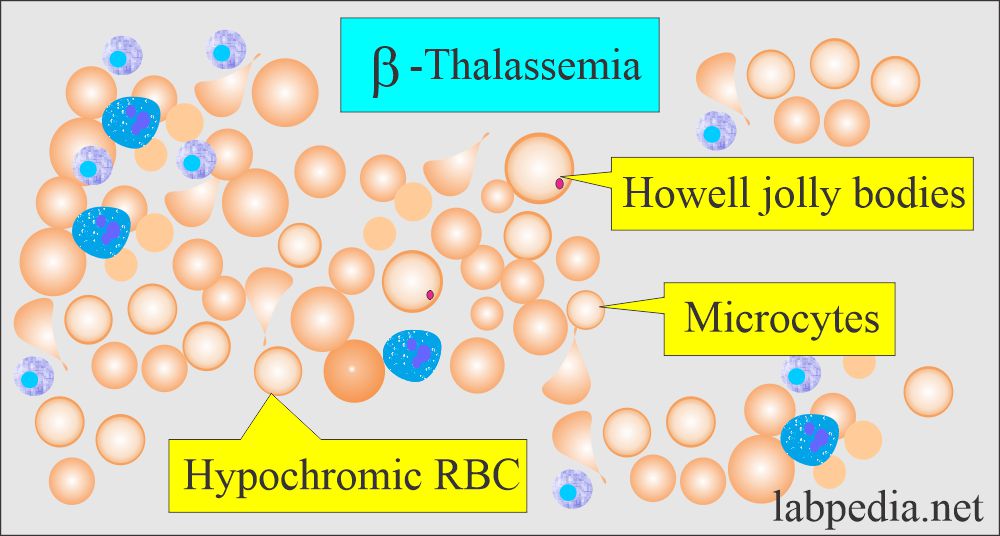
- The peripheral blood smear is typical of microcytic and hypochromic anemia.
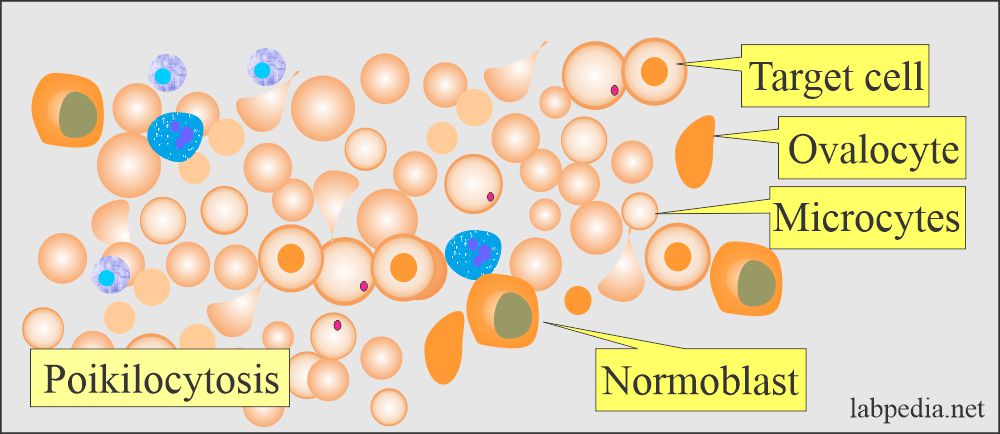
- In the case of homozygous β-thalassemia and double heterozygous non-α- thalassemia, peripheral smear shows:
- Severe anisocytosis.
- Poikilocytosis with bizarre shape.
- There are target cells.
- There are ovalocytes.
- There are numerous nucleated RBCs.
- In heterozygous β-thalassemia, peripheral blood smear shows:
- There are hypochromic microcytic RBCs with moderate anisocytosis and poikilocytosis.
- Basophilic stippling is also seen.
- MCV is the best screening for patients with thalassemia. There is decreased MCV, 85% chance if this is <75 fl (femtoliters).
- RBC count of >5 million/mm3.
- Hemoglobin (Hb) level is <9 g/dL.
- MCHC <30%.
- Reticulocytes are increased in these patients. This is increased in:
- In Hb H, the disease may be up to 10%.
- In homozygous β-thalassemia may reach 5%.
- Normal RDW (red cell volume distribution width).
- The only exception of RDW in Thalassemia major is the degree of anisocytosis leading to an increased level.
- In iron deficiency, the RDW is increased, and the decrease in MCV is less striking.
Differential diagnoses of various anemias:
| Type of anemia | Serum iron | TIBC | Ferritin level | RDW | Free RBC protoporphyrin | A2 level |
| α-thalassemia | Normal | Normal | Normal | Normal | Normal | Normal |
| β-thalassemia | Normal | Normal | Normal | Normal | Normal | Increased |
| Iron-deficiency anemia | Decreased | Increased | Decreased | Increased | Increased | Normal |
| Chronic diseases anemia | Decreased | Decreased | Increased | Normal | Increased | Normal |
- In heterozygous thalassemia, MCH is <22 pg, MCV <70 fl, and Hb is around 9 to 11 g/dL.
- Cord blood can be used to diagnose thalassemia.
- DNA analysis of chorionic villus sample at 8 to 10 weeks of pregnancy.
- Or Amniotic fluid cells by amniocentesis at 16 to 18 weeks.
- Serum electrophoresis of the infants.
- Electrophoresis plays an important role in diagnosing thalassemia, and it will detect an increased level of HbA2, HbF, and other abnormal hemoglobins (HbH and Bart’s).
The following table shows β-thalassemia hemoglobin on Hb-electrophoresis:
| Type of thalassemia | HbA | HbA2 | HbF |
| Normal infants | 95% to 97% | 2% to 3% | 1% to 2% |
| β-thalassemia homozygous type | Nil | 2% to 5% | 95% to 98% |
| β-thalassemia Heterozygous | 90% to 95% | 3.5% to 7% | 2% to 5% |
| β+-thalassemia homozygous or double heterozygous β+/β0 | 5% to 35% | 2% to 5% | 60% to 95% |
| Heterozygousα δβ- thalassemia (Hb Lepore syndrome) | 80% to 92% | 1% to 2.5% | 5% to 20% |
| Hereditary persistent HbF (HPHF) (homozygous) | Nil | Nil | 100% |
| HPHF heterozygous African type | 65% to 85% | 1% to 2.5% | 15% to 35% |
| HPHF heterozygous Greek type | 755 to 85% | 1.5% to 2.5% | 15% to 25% |
Summary of thalassemia:
Questions and answers:
Question 1: What is Bart's hemoglobin?
Question 2: What is hemoglobin H (Hb H)?