Electrolytes:- Part 1 – Potassium (K+) Blood and its Significance
Electrolytes
Potassium (K+) Blood
What ype of Sample for Potassium (K+) is needed?
- This is done on the patient’s serum.
- Separate serum as soon as possible.
- Plasma can be used, but it gives slightly lower values.
- A random sample may be taken.
- Serum or plasma is stable for one week at room temperature or 1°C to 4 °C.
What are the Precautions for Potassium (K+)?
- Avoid hemolysis, which may increase the value.
- Avoid prolonged tourniquet time or repeated fist clenching during venipuncture, as these can increase potassium levels.
- Increased value of platelets or white blood cell counts will increase the value.
- EDTA should not be used because it contains K+.
- Serum or plasma should be separated within 3 hours to prevent leakage of the K+ from the blood cells.
- Incomplete separation of serum and clot.
- Excess food intake or rapid potassium I/V therapy.
- Drugs with high potassium contents, like penicillin G.
- Transfusion of the old stored blood.
What are the Indications for Potassium (K+)?
- Potassium is part of the electrolyte estimation.
- Potassium is advised for all serious patients.
- Potassium is advised for patients receiving diuretics or heart medication.
How will you define Potassium?
- It is mainly an intracellular ion.
- <2% is present in the extracellular space.
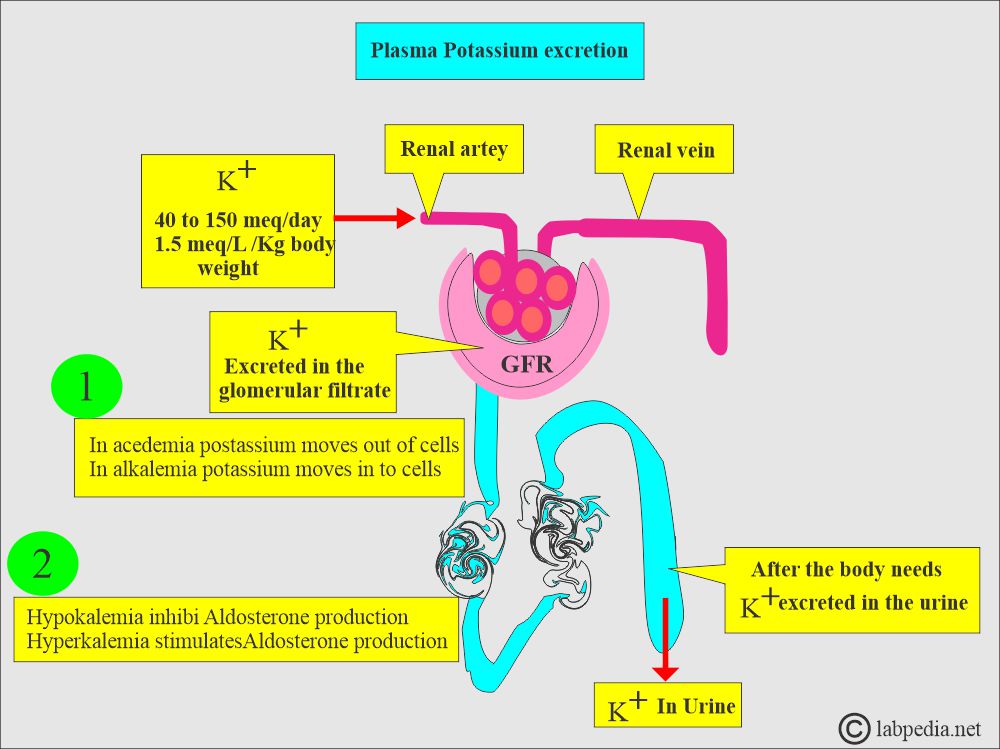
- In acedemia, potassium moves out of the cells.
- In alkalemia, potassium moves into the cells.
- Hypokalemia inhibits aldosterone production.
- Hyperkalemia stimulates aldosterone production.
- Plasma sodium and potassium control potassium reabsorption.
How will you describe the Pathophysiology of Potassium (K+)?
- Potassium is the main electrolyte of intracellular fluid.
- <2% are extracellular.
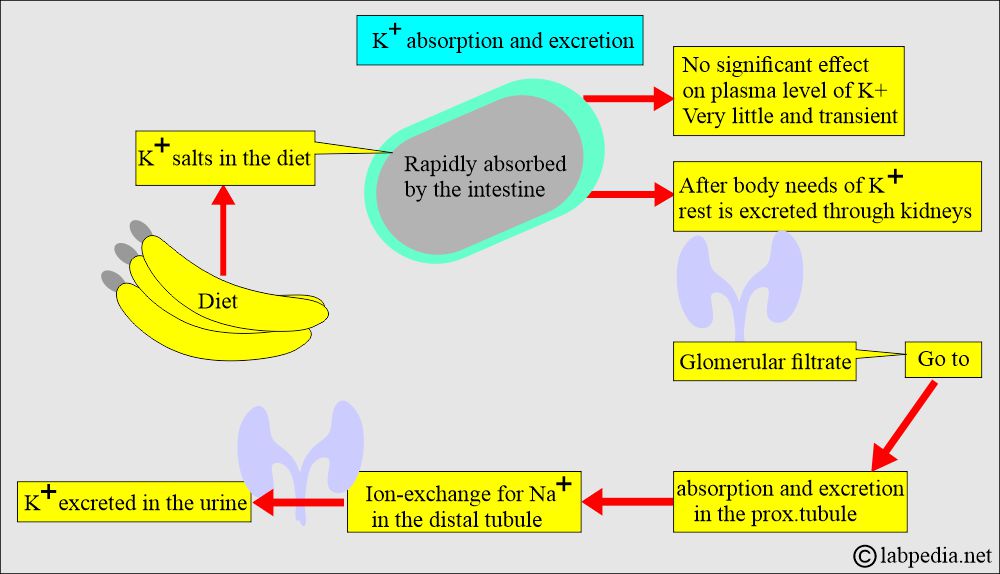
- About 2 to 3 grams of potassium is ingested in the food and excreted as salts.
- The intestine rapidly absorbs potassium salts.
- There is very little effect on the plasma level.
- After the body needs potassium, it is excreted through the kidneys.
- The daily potassium intake is 40 to 150 meq/day, with and average of 1.5 meq/Kg body weight.
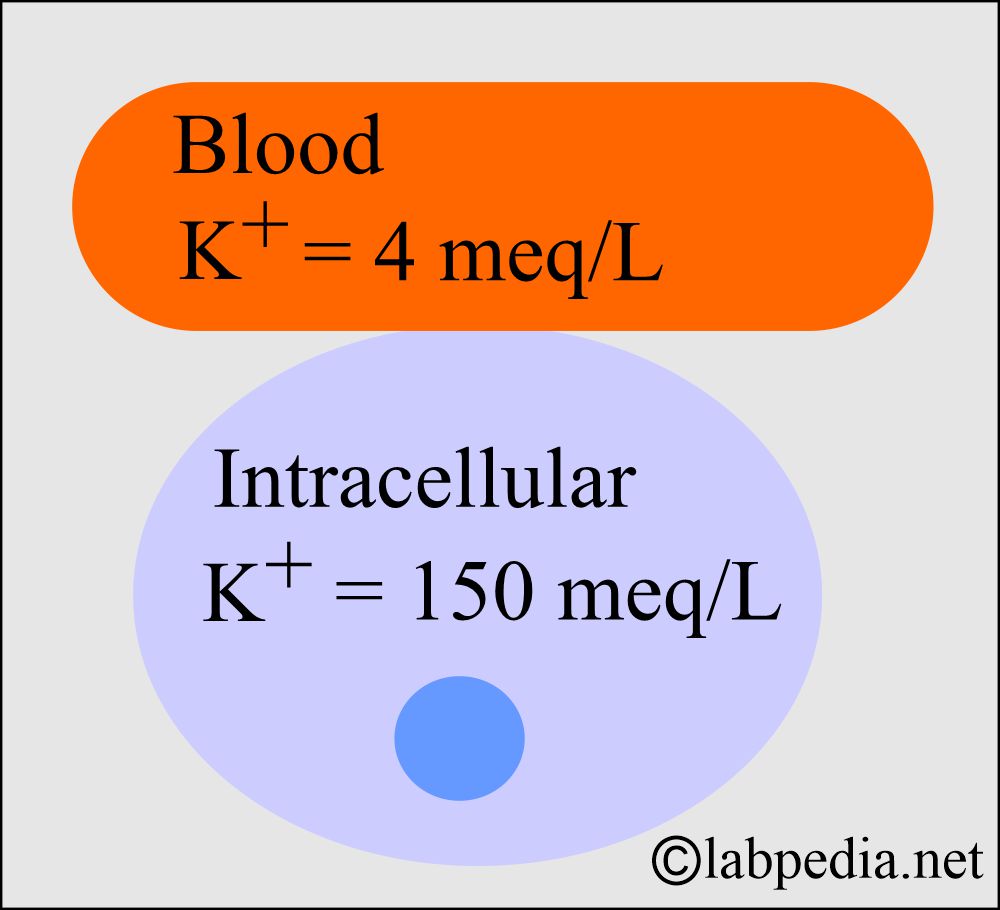
- The intracellular Potassium is 150 meq/L, and in the blood is approximately 4 meq/L.
- This intracellular and extracellular potassium ratio is crucial to maintaining the membrane electrical potential.
- Potassium is the primary buffer system in the cells.
- The main concentration of potassium is within the cell, almost 90%.
- A very small amount is present in the blood and bone.
- When the cells are damaged, potassium is released into the blood and may give rise to an increased value.
How will you describe the Excretion of Potassium?
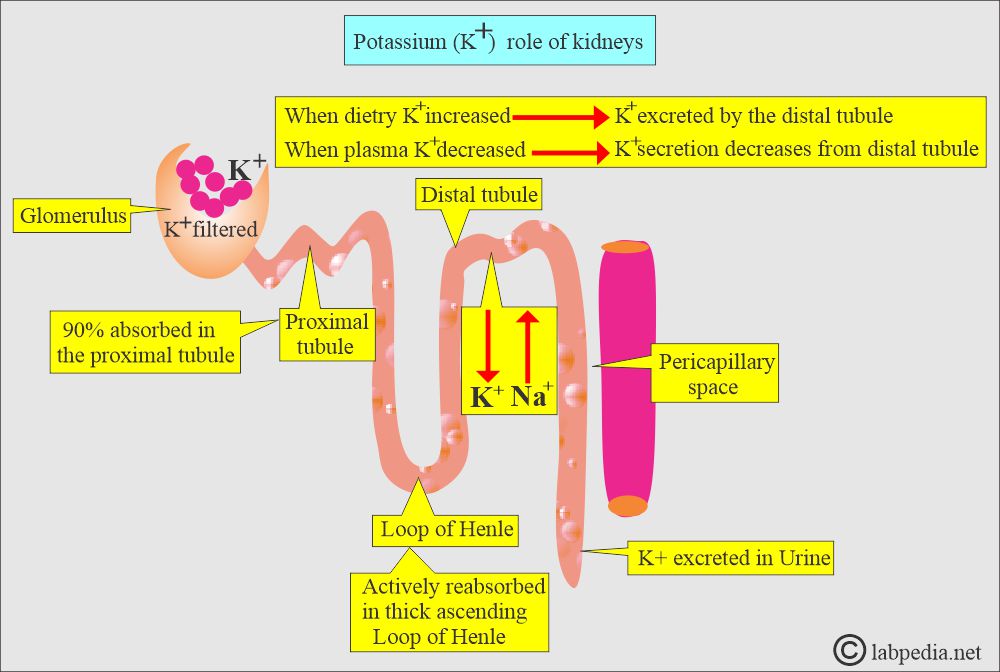
- 80% to 90% of Potassium is excreted by the glomeruli in the urine (filtered at the glomerulus).
- Reabsorbed passively in the proximal tubules and actively in the thick ascending loop of Henle.
- Secreted or actively reabsorbed in the distle convoluted tubules or collecting ducts, depending upon the potassium blood level.
- A lesser amount of 10% to 20% is excreted in the sweat and stool.
- Potassium’s role in the body is vital.
| The site of K+ loss | K+ loss |
|
|
|
|
|
|
- Kidneys do not conserve potassium, so potassium may be deficient when intake is decreased.
- A normal adult needs 80 to 200 meq /day of potassium in the diet.
What are the factors on which the Potassium concentration depends?
- Hormonal effect where aldosterone and, to some extent, glucocorticoids increase Renal Potassium loss.
- Absorption of Sodium: When Sodium is reabsorbed, then Potassium is lost.
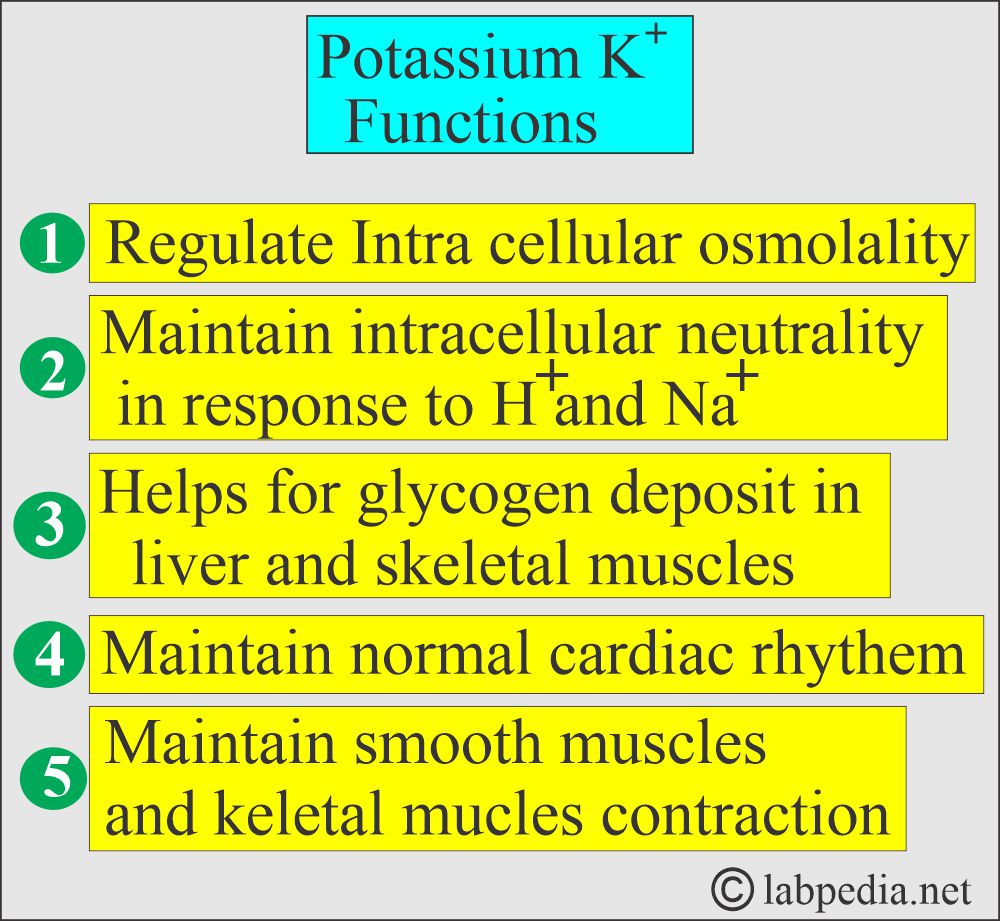
What are the functions of Potassium (K+)?
- Most potassium is found within cells (intracellular), significantly influencing the conduction of electrical impulses in cardiac and skeletal muscles.
- Potassium plays a vital role in the following:
- Nerve conduction.
- Muscular function.
- Osmotic pressure.
- Protein synthesis.
- Acid-base balance.
- In numerous enzyme reactions of carbohydrate and protein metabolism.
- Potassium, calcium, and magnesium control cardiac output, heart muscle contraction, and heart rate.
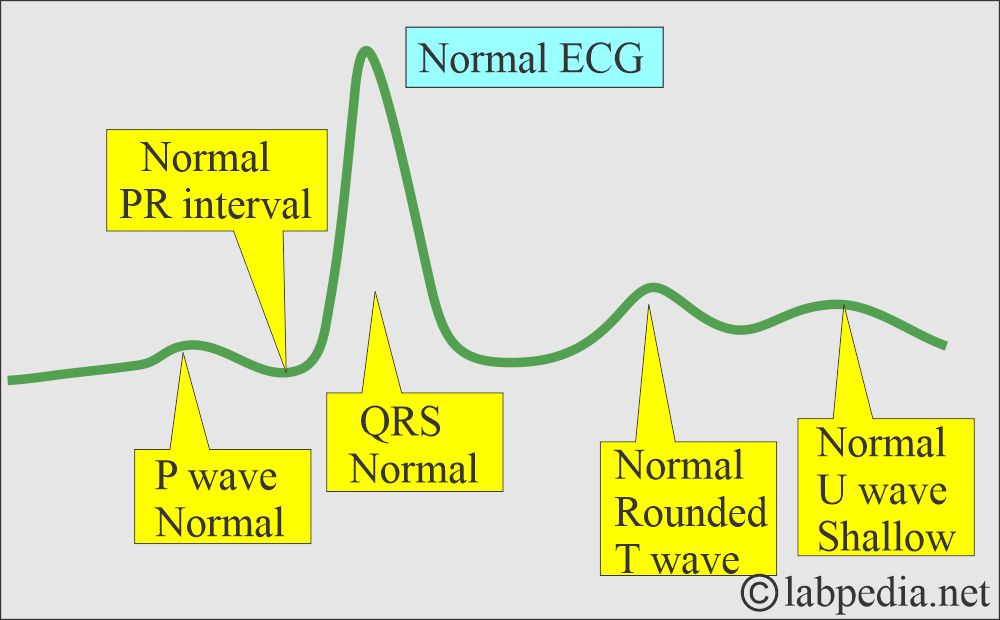
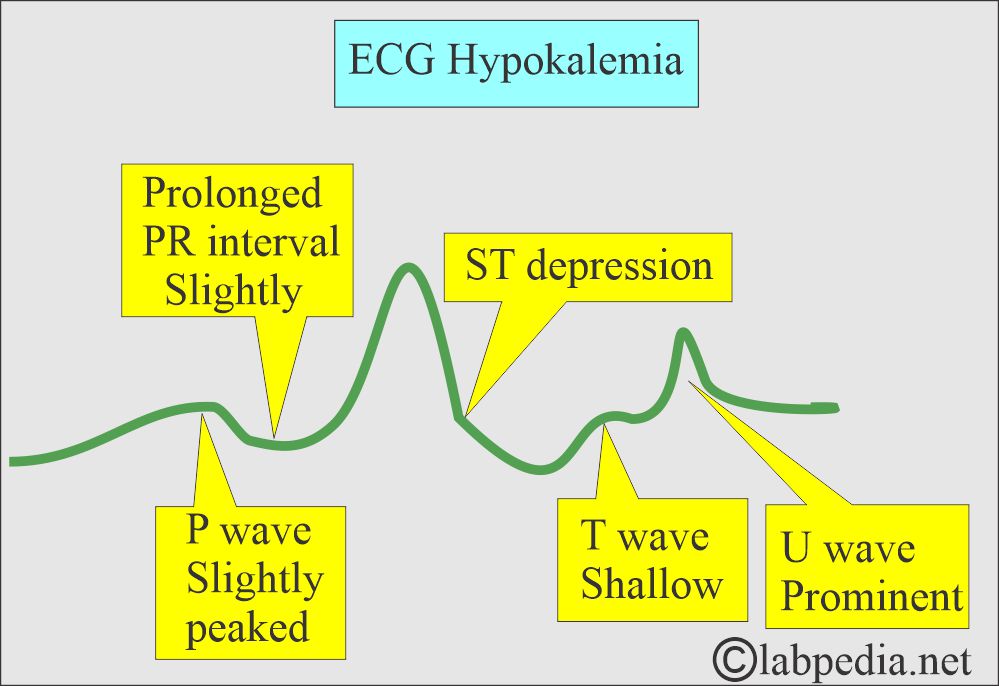
- Potassium deficiency on the ECG shows a U wave.
What is the role of Potassium (K+) in acid-base balance?
- H+ ions replace Potassium and Sodium in the renal tubules.
- Potassium is more important than sodium.
- Potassium bicarbonate (K+HCO3–) is the only intracellular inorganic buffer.
- In Potassium deficiency, in other words, there is a decrease in HCO3–, so pH will be relatively acidic.
- Now, the respiratory center is stimulated by low pH and by a decrease in pCO2 from hyperventilation.
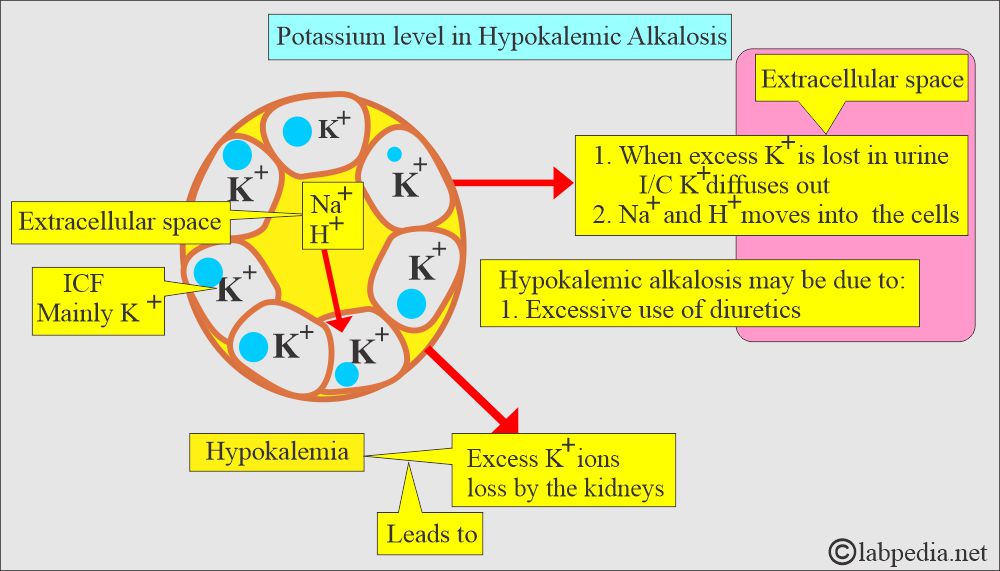
What is Hypokalemic alkalosis?
- Serum potassium is lowered by shifting potassium into cells.
- The excess excretion usually results in potassium loss by the kidneys into the urine, as seen with excessive diuretic use, which causes potassium and sodium loss.
- When excess potassium is lost in the urine, intracellular potassium diffuses into the plasma to replace some of the potassium lost from the plasma.
- Sodium (Na+) and hydrogen (H+) ions move into the cells to replace the K+ that have moved out.
What are the Lab findings in hypokalemic Alkalosis?
- Increased plasma pH.
- Decreased ECF (Extracellular fluid) hydrogen ion concentration.
- Increased K+ excretion in exchange for the urinary Na+ leads to hypokalemia.
- Hypokalemia results from the depletion of the intracellular K+.
- HCO3– level is increased.
- Serum chloride level is decreased.
- In some cases, we may see low serum Na+.
- ECG changes are typical.
What is Hyperkalemic acidosis?
- In acidosis, potassium (K+) moves from cells into the blood. This is a reverse phenomenon.
- Release from cells exceeds excretion by the kidneys. This occurs in acidosis and anoxia.
What are the lab findings in hyperkalemic acidosis?
- K+ level is raised.
- Low blood pH.
- HCO3– level is mostly low.
- Increased anion gap.
- Decreased blood CO2 level.
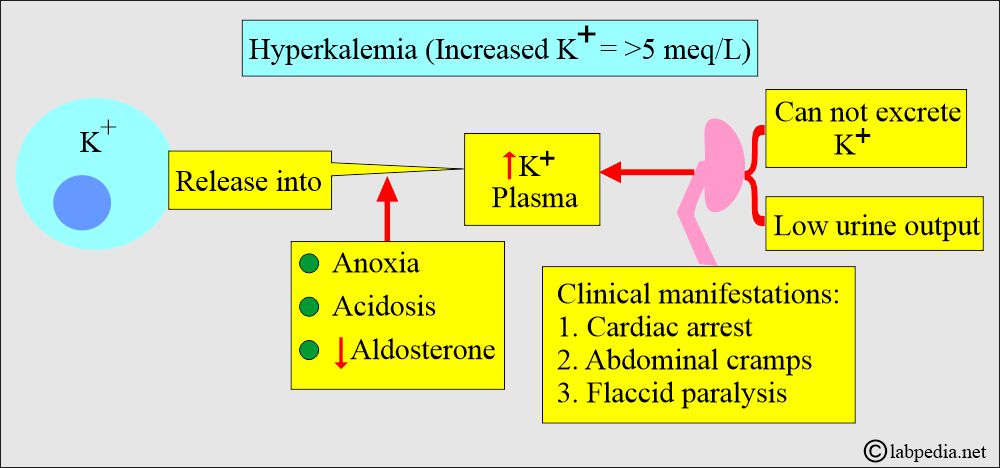
What causes Hyperkalemia (Increased concentration of K+ in the blood)?
- This is due to the following:
- Increased potassium is released into the blood.
- Or due to the kidney, which cannot excrete the potassium.
- Or due to low urine output.
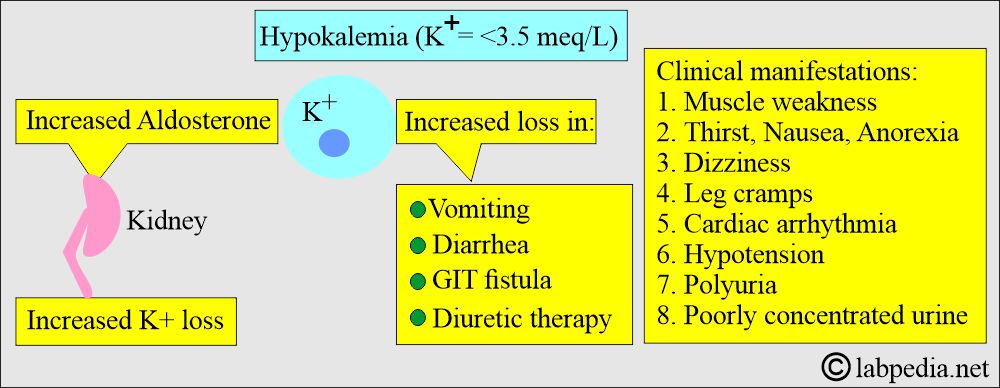
What causes Hypokalemia (Decreased concentration of K+) in the blood?
- This is due to potassium loss in vomiting, diarrhea, GIT fistula, and diuretics.
- Aldosterone increases lead to a decrease in potassium.
What are the Signs and Symptoms of Potassium (K+) level changes in the body?
- The S/S depends upon the concentration of the K+ in the blood.
- Potassium level <2.5 meq/L
- There will be tachycardia.
- There is increased muscular irritability.
- There are specific cardiac conduction defects.
- There is a stoppage of the heart in the systole.
- There is a flattened T-wave.
- The end result will be cardiac arrest.
- Potassium level <3.0 meq/L
- There are marked neuromuscular symptoms.
- Potassium level >6.5 meq/L
- There is peripheral vascular collapse.
- Inhibit muscle irritability.
- Ultimately, cardiac arrest and the stoppage of a heartbeat.
What are the S/S due to Hyperkalemia?
- There is mental confusion.
- There is a weakness.
- There is a tingling sensation.
- Flaccid paralysis of limbs.
- There is a weakness in the respiratory muscles.
- There is bradycardia.
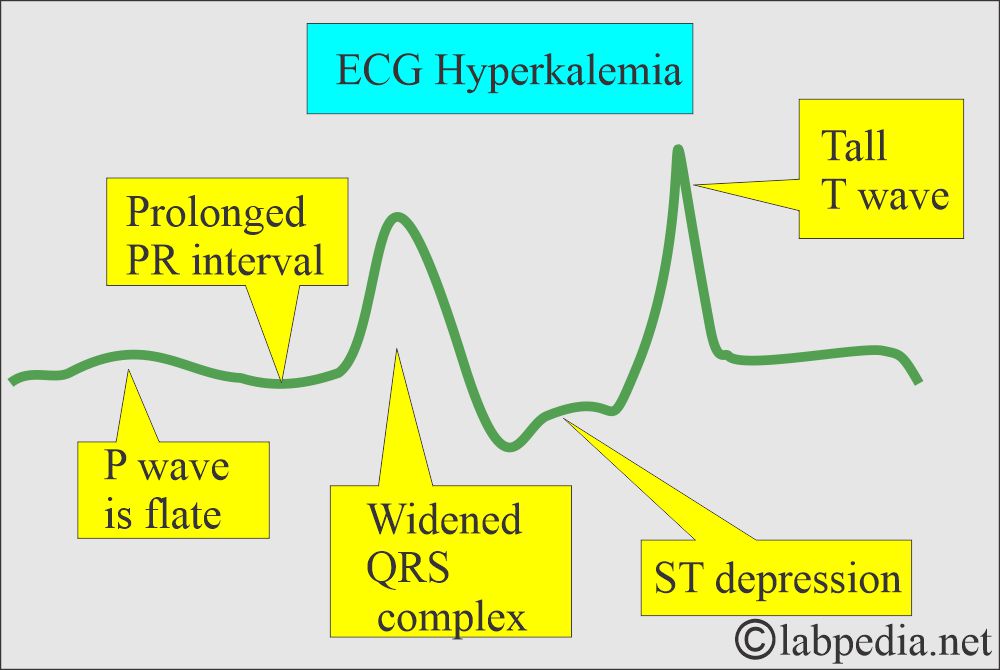
- There are prolonged PR and QRS intervals.
- There is a peaked T-wave.
What are the Critical values of K+?
- Potassium level >6.5 meq/L
- There is peripheral vascular collapse.
- Inhibit muscle irritability.
- Ultimately, cardiac arrest and the stoppage of a heartbeat.
- Potassium level <2.5 meq/L
- Cardiac electrical activity can be seriously altered with arrhythmias.
- The potassium level of 10.0 meq/L
- It is fatal to the patient’s life and stops the cardiac activity.
What are the NORMAL values of Potassium (serum)?
Source 1
| Age | meq/L |
| Premature cord blood | 5.0 to 10.2 |
| Premature by 48 hours | 3.6 to 6.0 |
| Newborn cord | 5.6 to 12.0 |
| Newborn | 3.7 to 5.9 |
| Infant | 4.1 to 5.3 |
| Child | 3.4 to 4.7 |
| Adult | 3.5 to 5.1 |
- To convert to SI units x 1.0 = mmol/L
Source 2
- Adult = 3.5 to 5.0 meq/L
Child = 3.4 to 4.7 meq/L - Infants = 4.1 to 5.3 meq/L.
- Newborn = 3.9 to 5.9 meq/L.
- Urine = 25 to 125 meq /day.
- CSF = 2.2 to 3.3 meq/L
What is the difference between serum and blood (plasma) K+ levels?
- Serum K+ level is higher 0.4 to 0.5 meq/L from the blood (plasma).
- The literature range is 0.1 to 1.2 meq/L.
- Na+ level is the same in serum, blood, and plasma.
What causes Hyperkalemia (increased serum Potassium level)?
- Increased dietary uptake.
- Acute and chronic renal failure.
- Addison’s disease.
- Decreased Aldosterone and hypoaldosteronism.
- Hemolysis.
- Transfusion of hemolyzed blood.
- Uncontrolled diabetes mellitus.
- Metabolic acidosis.
- In Burns, accidents, surgery, chemotherapy, and DIC.
- Kidney transplant rejection.
- Decreased excretion of potassium in the urine:
- Renal failure.
- Acidosis.
- Adrenocortical insufficiency.
What are the ECG changes in hyperkalemia?
- The T-wave is elevated.
- P-wave is flattened.
- Cardiac arrest may occur without warning of any other changes.
- Nearly all cases of acidosis are associated with hyperkalemia.
What are the causes of Hypokalemia (decreased Potassium)?
- Decreased dietary intake.
- Dehydration.
- Acidosis.
- Increased gastrointestinal losses, such as diarrhea and vomiting.
- Excessive sweating.
- Starvation and malnutrition.
- Cystic fibrosis.
- Severe burns.
- Respiratory alkalosis.
- Renal tubular acidosis.
- Respiratory alkalosis.
- Diuretics.
- Hyperaldosteronism.
- Cushing syndrome.
- Trauma due to surgery or burns.
- Gastrointestinal losses include vomiting, nasogastric tube, diarrhea, and villous adenoma.
- Renal losses such as diuretics, antibiotics (ampicillin-B and carbenicillin), hypomagnesemia, renal tubular acidosis, mineralocorticoid excess, congenital adrenal hyperplasia, and Cushing’s syndrome.
- There may be transcellular shifts, like alkalosis and correction of diabetic ketoacidosis.
What are the ECG Changes in hypokalemia?
- T-waves are depressed.
- P-wave has peaked.
- ST – depression.
- U-wave is prominent.
What will happen in case of K+ loss in Non-renal patients?
What are the Causes of non-renal potassium loss?
- These patients have hypokalemia, and urinary potassium is <25 meq/24 hours or <15 meq/L due to extra-renal causes.
- The causes are:
- Vomiting.
- Diarrhea due to infections, malabsorption, or radiation.
- Neoplasms like villous adenomas of the colon and Zollinger-Ellison syndrome.
- Excessive spitting in neurotic patients.
- Excessive sweating.
- Cystic fibrosis.
- Excessive burns.
- Respiratory alkalosis.
- Accidental absorption of barium compounds.
- Dietary deficiency.
What will be a presentation of Pseudohyperkalemia?
- Potassium is elevated, and there are no clinical changes in cardiac excitability.
- The ECG can confirm this.
- In these patients, no treatment is needed, and in fact, this may be harmful.
- In such cases, the potassium may be released in the following conditions:
- In vitro hemolysis.
- In vitro clot formation.
- Thrombocytosis.
- Leukocytosis.
- Due to tourniquet use.
What are the Potassium (K+) levels in blood and urine in various conditions?
| Various clinical conditions | Potassium (K+) level in the blood | Potassium (K+) level in urine |
| Diarrhea | Decreased | Normal or decreased |
| Dehydration | Increased | Increased |
| Malabsorption | Decreased | Decreased |
| Starvation | Decreased | Increased or normal |
| Excessive sweating | Normal | Normal |
| Pyloric obstruction | Decreased | Normal |
| Congestive heart failure | Normal | Normal |
| Pulmonary emphysema | Normal | Normal |
| Acute renal failure | Increased | Decreased |
| Chronic renal failure | Normal or decreased | Increased |
| Renal tubular acidosis | Decreased | Increased |
| Primary aldosteronism | Decreased | Increased |
| Adrenal cortical insufficiency | Increased | Normal or decreased |
| Diabetic acidosis | Normal or increased | Increased |
| Diabetes inspidus | Normal | Normal |
| Thiazide diuretics | Decreased | Increased |
| Mercurial diuretics | Decreased | Increased |
| Diamox (Acetazolamide) | Decreased | Increased |
- Note: Please see the Electrolytes section for more details.
Questions and answers:
Question 1: What are the changes in ECG in hypokalemia?
Question 2: What are the changes in hyperkalemia?